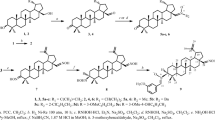
Abstract
The reactions of terpene nitrosochlorides derived from 3-carene, α-pinene, and limonene, with simplest azaheterocycles (imidazole, benzotriazole, and indole) were studied. On the base of these transformations, preparative procedures to access chiral oximes bearing azaheterocyclic moieties in the α-position to the oxime fragment, namely, α-(1H-imidazol-1-yl)-, α-(1H-benzo-[d][1,2,3]triazol-1-yl)-, and α-(1H-indol-3-yl)-substituted terpenic oximes, were developed. Transformations of the studied monoterpene nitrosochlorides into α-substituted oximes proceeded stereoselectively to give in the moderate yields (30–60%) the only stereoisomer arising from the attack of the heterocyclic anion from the less hindered side of the intermediate nitroso olefin generated in situ from nitrosochloride.
Similar content being viewed by others
References
A. V. Tkachev, Ross. Khim. Zh., 1998, 42, 42 [Mendeleev Chem. J. (Engl. Transl.), 1998, 42].
S. V. Larionov, A. V. Tkachev, Ross. Khim. Zh., 2004, 48, 154 [Mendeleev Chem. J. (Engl. Transl.), 2004, 48].
S. N. Bizjaev, T. V. Rybalova, Yu. V. Gatilov, A. V. Tkachev, Mendeleev Commun., 2004, 14, 18.
N. B. Gorshkov, A. M. Agafontsev, A. V. Tkachev, Izv. Akad. Nauk, Ser. Khim., 2010, 1434 [Russ. Chem. Bull., Int. Ed., 2010, 59, 1467].
A. V. Tkachev, A. V. Rukavishnikov, T. O. Korobeinicheva, Yu. V. Gatilov, I. Yu. Bagrjanskaja, Zh. Org. Khim., 1990, 26, 1939 [Russ. J. Org. Chem. (Engl. Transl.), 1990, 26].
A. V. Tkachev, A. M. Chibiryaev, A. Yu. Denisov, Yu. V. Gatilov, Tetrahedron, 1995, 51, 1789.
G. Widmark, Arkiv Kemi, 1957, 11, 195.
C. Bordenca, R. K. Allison, P. H. Dirstine, Ind. Eng. Chem., 1951, 43, 1196.
Yu. G. Putsykin, V. P. Tashchi, A. F. Rukasov, Yu. A. Baskakov, V. V. Negrebetskii, L. Ya. Bogel’fer, Zh. Vsesoyuz. Khim. Obshch. im. D. I. Mendeleeva, 1979, 24, 652 [Mendeleev Chem. J. (Engl. Transl.), 1979, 24].
A. V. Tkachev, A. V. Rukavishnikov, Yu. V. Gatilov, I. Yu. Bagrjanskaja, Zh. Org. Khim., 1990, 26, 1693 [J. Org. Chem. USSR (Engl. Transl.), 1990, 26, No. 8].
A. V. Tkachev, A. Yu. Denisov, A. V. Rukavishnikov, A. M. Chibirjaev, Yu. V. Gatilov, I. Yu. Bagrjanskaja, Aust. J. Chem., 1992, 45, 1077.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 587–593, March, 2012.
Rights and permissions
About this article
Cite this article
Bizyaev, S.N., Tkachev, A.V. Reactions of 3-carene, limonene, and α-pinene nitrosochlorides with imidazole, benzotriazole, and indole. Russ Chem Bull 61, 589–595 (2012). https://doi.org/10.1007/s11172-012-0085-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0085-7