Abstract
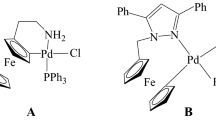
Two ferrocenyl palladacycles with bi-and tridentate (C,N) and (C,N,N) ligands were tested as possible catalysts for the Heck reaction. The latter complex efficiently catalyzed reactions of aryl halides with ethyl acrylate.
Similar content being viewed by others
References
Y. J. Wu, J. Hou, H. Yun, X. Cui, R. Yuan, J. Organomet. Chem., 2001, 637–639, 793.
C. Hu, J. F. Gong, S. F. Yue, Y. J. Wu, J. Chem. Soc., Dalton Trans., 2006, 4730.
B. Mu, T. Li, W. Hu, G. Zeng, P. Liu, Y. J. Wu, Tetrahedron, 2007, 63, 11475.
L. L. Troitskaya, V. I. Sokolov, O. A. Reutov, Dokl. Akad. Nauk SSSR, 1979, 246, 124 [Dokl. Chem. (Engl. Transl.), 1979, 246].
N. S. Khrushcheva, V. I. Sokolov, Izv. Akad. Nauk, Ser. Khim., 2006, 1116 [Russ. Chem. Bull., Int. Ed., 2006, 55, 1159].
L. A. Bulygina, V. I. Sokolov, I. A. Utepova, V. L. Rusinov, O. N. Chupakhin, Izv. Akad. Nauk, Ser. Khim., 2007, 1039 [Russ. Chem. Bull., Int. Ed., 2007, 56, 1080].
V. I. Sokolov, L. L. Troitskaya, O. A. Reutov, J. Organomet. Chem., 1979, 182, 537.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1370–1372, July, 2010.
Rights and permissions
About this article
Cite this article
Sokolov, V.I., Bulygina, L.A., Khrushcheva, N.S. et al. Use of ferrocenyl chelated palladacycles as catalysts for the Heck reaction. Russ Chem Bull 59, 1400–1402 (2010). https://doi.org/10.1007/s11172-010-0253-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0253-6