Abstract
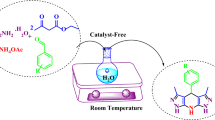
The present work describes the applications of Brönsted acid hydrotrope combined catalyst (BAHC) as a mild, efficient and reusable catalyst for synthesis of indoloquinoxalines and bis-tetronic acids in water. Using BAHC, we synthesized many indoloquinoxaline derivatives from isatins and o-phenylene diamine using 10 mol% PTSA in 40% aqueous hydrotropic (NaPTS) solution at room temperature with 83–90% yields. On the other hand, the reaction of tetronic acid with the aldehydes/isatins forms bis-tetronic acids with 83–88% yields through Knoevengel condensation-Michael addition pathway in same BHAC. Moreover, the BAHC can be recycled upto 5th cycles with slight decrease in product yields. The extremely simple operational methodology, green solvent, ambient reaction conditions and high yields render this approach extremely appealing for the synthesis of different heterocyclic compounds.





Similar content being viewed by others
Change history
26 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11164-021-04495-7
References
C.-J. Li, L. Chen, Chem. Soc. Rev. 35, 68 (2006)
V.R. Vemula, V. Lagishetty, S. Lingala, Int. J. Pharm. Sci. Rev. Res. 5, 41 (2010)
C. Neuberg, Biochem. Z. 76, 107 (1916)
N.S. Tavare, V.K. Jadhav, J. Chem. Eng. Data. 41, 1196 (1996)
V. Srinivas, D. Balasubramanian, Langmuir 14(23), 6658 (1998)
V. Srinivas, G.A. Rodley, K. Ravikumar, W.T. Robinson, M.M. Turnbull, Langmuir 13(12), 3235 (1997)
A. Sapkal, S. Kamble, J. Heterocycl. Chem. 57(10), 3597 (2020)
G. Rashinkar, S. Kamble, A. Kumbhar, R. Salunkhe, Trans. Met. Chem. 35, 185 (2010)
S. Kamble, A. Kumbhar, G. Rashinkar, M. Barge, R. Salunkhe, Ultrason Sonochem. 19(4), 812 (2012)
M. Barge, S. Kamble, A. Kumbhar, G. Rashinkar, R. Salunkhe, Monatsh. Chem. 144(8), 1213 (2013)
S. Jadhav, A. Kumbhar, C. Rode, R. Salunkhe, Green Chem. 18, 1898 (2016)
A. Patil, R. Salunkhe, Res. Chem. Intermed. 43, 4175 (2017)
A. Patil, M. Barge, G. Rashinkar, R. Salunkhe, Mol. Divers. 19(3), 435 (2015)
R. Pratap, V.J. Ram, Chem. Rev. 114(20), 10476 (2014)
C.H. Jin, M. Krishnaiah, D. Sreenu, V.B. Subrahmanyam, H.-J. Park, S.-J. Park, Y.Y. Sheen, D.-K. Kim, Bioorg. Med. Chem. 22(9), 2724 (2014)
D. Zych, A. Slodek, S. Krompiec, K. Malarz, A. Mrozek-Wilczkiewicz, R. Musiol, Chem. Select 3(24), 7009 (2018)
P.K. Paliwal, S.R. Jetti, S. Jain, Med. Chem. Res. 22(6), 2984 (2013)
Q. Li, S. He, Y. Chen, F. Feng, W. Qu, H. Sun, Eur. J. Med. Chem. 158, 463 (2018)
X. Wan, C. Li, M. Zhang, Y. Chen, Chem. Soc. Rev. 49, 2828 (2020)
A. Slodek, D. Zych, A. Maroń, S. Golba, E. Schab-Balcerzak, H. Janeczek, M. Siwy, S. Maćkowski, Dyes Pigm. 166, 98 (2019)
H. Lai, J. Hong, P. Liu, C. Yuan, Y. Li, Q. Fang, RSC Adv. 2, 2427 (2012)
Y. Feng, D. Li, Q. Wang, S. Wang, X. Meng, Z. Shao, M. Zhu, X. Wang, Sens. Actuators B Chem. 225, 572 (2016)
A. Slodek, D. Zych, S. Golba, S. Zimosz, P. Gnida, E. Schab-Balcerzak, J. Mat. Chem. C. 7, 5830 (2019)
M. Judith Percino, M. Cerón, P. Venkatesan, E. Pérez-Gutiérrez, P. Santos, P. Ceballos et al., RSC Adv. 9, 28704 (2019)
M.S. Abdelfattah, T. Kazufumi, M. Ishibashi, J. Nat. Prod. 73(12), 1999 (2010)
P.S. Chandrachood, A.R. Jadhav, D.R. Garud, N.R. Deshpande, V.G. Puranik, R.V. Kashalkar, Res. Chem. Intermed. 46, 5219 (2020)
G. Sakata, K. Makino, Y. Kurasawa, Heterocycles 27, 2481 (1988)
K.R. Justin Thomas, M. Velusamy, J.T. Lin, C.H. Chuen, Y.T. Tao, Chem. Mater. 17(7), 1860 (2005)
S. Dailey, J.W. Feast, R.J. Peace, I.C. Sage, S.Till, E.L.Wood, J. Mater. Chem. 11, 2238 (2001)
M.J. Crossley, L.A. Johnston, Chem. Commun. 1122 (2002)
M.M. Abdou, R.A. El-Saeed, M.A. Abozeid, M.G. Sadek, E. Zaki, Y. Barakat, H. Ibrahim, M. Fathy, S. Shabana, M. Amine, S. Bondock, Arab. J. Chem. 12(4), 464 (2019)
A.L. Zografos, D. Georgiadis, Synthesis 3157 (2006)
D. Tejedor, F. García-Tellado, Org. Prep. Proced. Int. 36(1), 33 (2004)
L. Vieweg, S. Reichau, R. Schobert, P.F. Leadlay, R.D. Süssmuth. Nat. Prod. Rep. 31(11), 1554 (2014)
B.E. Roggo, F. Petersen, R. Delmendo, H.B. Jenny, H.H. Peter, J. Roesel, J. Antibiot. 47(2), 136 (1994)
K. Rehse, U. Emisch, Arch. Pharm. 316(2), 115 (1983)
K. Luk, S.A. Readshaw, J. Chem. Soc. Perkin Trans. 1(7), 1641 (1991)
E.J. Murray, R.C. Crowley, A. Truman, S.R. Clarke, J.A. Cottam, G.P. Jadhav, V.R. Steele, P. O’Shea, C. Lindholm, A. Cockayne, S.R. Chhabra, W.C. Chan, P. Williams, J. Med. Chem. 57(6), 2813 (2014)
A. Dal Pozzo, A. Dansi, E. Neneghini, Bull. Chim. Farm. 113(5), 280 (1974)
F.R. Foden, J. McCormick, D.M. O’Mant, J. Med. Chem. 18(2), 199 (1975)
M. Andreoli, M. Persico, A. Kumar, N. Orteca, V. Kumar, A. Pepe, C. Fattorusso, et al., J. Med. Chem. 57(19), 7916 (2014)
A. Kumbhar, S. Kamble, M. Barge, G. Rashinkar, R. Salunkhe, Tetrahedron Lett. 53(22), 2756 (2012)
R. Jain, K. Sharma, D. Kumar, Tetrahedron Lett. 53(46), 6236 (2012)
R. Dowlatabadi, A. Khalaj, S. Rahimian, M. Montazeri, M. Amini, A. Shahverdi, E. Mahjub, Synth. Commun. 41, 1650 (2011)
P.G. Hegade, M.M. Mane, J.D. Patil, D.M. Pore, Syn. Commun. 44(23), 3384 (2014)
Z.Z. Zhang, H. Zhang, W.Z.-Q. Li, C.C. Zeng, R.-G. Zhong, Y.-B. She, RSC Adv. 1, 583 (2011)
M. Dabiri, Z.N. Tisseh, M. Bahramnejad, A. Bazgir, Ultrason. Sonochem. 18, 353 (2011)
K.S. Pandit, U.V. Desai, P.P. Wadgaonkar, K.M. Kodam, Res. Chem. Intermed. 43, 141 (2016)
Acknowledgements
We like to show our appreciation to the University Grants Commission, Government of India, New Delhi, for enduring this work under the scheme of Major Research Project [F.No.41-182/2014 (SR)]. We also thankful to the Department of Chemistry, Shivaji University, Kolhapur for providing IR, 1H and 13C NMR Spectral analytical facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The citation “20” was incorrectly linked to the DOI link “https://doi.org/10.1016%2Fj.dyepig.2019.03.028”. However, the correct DOI link should be “https://doi.org/10.1016/j.dyepig.2019.03.032”
Rights and permissions
About this article
Cite this article
Kumbhar, A., Kanase, D., Mohite, S. et al. Brönsted acid hydrotrope combined catalysis in water: a green approach for the synthesis of indoloquinoxalines and bis-tetronic acids. Res Chem Intermed 47, 2263–2278 (2021). https://doi.org/10.1007/s11164-021-04430-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04430-w