Abstract
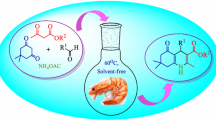
New 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones were synthesized, and good-to-excellent yields were achieved through one-pot, three-component condensation of aryl glyoxal monohydrates, 4-hydroxyquinolin-2(1H)-one and 3-nitroaniline using sodium alginate and water/ethanol (1:1) as a green solvent at mild reaction conditions. The noticeable features of the present procedure are mild reaction conditions, economic procedure, availability of starting materials, very simple operation, easy isolation, no need for column chromatography separation and using sodium alginate as a natural polysaccharide that is a transition-metal-free, biodegradable, reusable and commercially affordable catalyst. In addition, sodium alginate was recycled up to five times with no remarkable loss of its catalytic properties.
Graphic abstract





Similar content being viewed by others
References
B.H. Rotstein, V. Zaretsky, R. Yudia, A.K. Yudia, Chem. Rev. 114, 8323 (2014)
S.B. Azimi, J. Azizian, Tetrahedon Lett. 57, 181 (2016)
Z. Fu, K. Qian, S. Li, T. Shen, Q. Song, Tetrahedron Lett. 57, 1104 (2016)
S.R. Vidadala, H. Waldmann, Tetrahedron Lett. 56, 3358 (2015)
P.G. Jessop, Green Chem. 13, 1391 (2011)
R.A. Sheldon, Green Chem. 7, 267 (2005)
K. Shanab, C. Neudorfer, E. Schirmer, H. Spreitzer, Curr. Org. Chem. 17, 1179 (2013)
J. Su, M. Zhuang, F. Ma, W.Q. Zhang, H. Sun, G. Zhang, Y. Jian, Z. Gao, Asian J. Org. Chem. 8, 482 (2019)
J. Mao, Z. Wang, X. Xu, G. Liu, R. Jiang, H. Guan, Z. Zheng, P.J. Walsh, Angew. Chem. 131, 11149 (2019)
R. Lai, X. Wu, S. Lv, C. Zhang, M. He, Y. Chen, Q. Wang, Y. Wu, Chem. Commun. 55, 4039 (2019)
J. Pan, R. Zhao, J. Guo, D. Ma, Y. Xia, Y. Gao, P. Xu, Y. Zhao, Green Chem. 21, 792 (2019)
H.R. Hudson, N.J. Wardle, S.W. Bligh, I. Greiner, A. Grun, G. Keglevich, Mini-Rev. Med. Chem. 12, 313 (2012)
C. Queffélec, M. Petit, P. Janvier, D.A. Knight, B. Bujoli, Chem. Rev. 112, 3777 (2012)
C.S. Demmer, N. Krogsgaard-Larsen, L. Bunch, Chem. Rev. 111, 7981 (2011)
T.V. Sravanthi, S.L. Manju, Eur. J. Pharm. Sci. 91, 1 (2016)
I. Andreadou, A. Tasouli, E. Bofilis, M. Chrysselis, E. Rekka, A. Tsantili-Kakoulidou, E. Iliodromitis, T. Siatra, D.T. Kremastinos, Chem. Pharm. Bull. 50, 165 (2002)
C. Karaaslan, H. Kadri, T. Coban, S. Suzen, A.D. Westwell, Bioorg. Med. Chem. Lett. 23, 2671 (2013)
M.M. Sayed, Y.M. Moustafa, A.N. Mohamed, Med. Chem. Res. 23, 3377 (2014)
T.C. Leboho, J.P. Michael, W.A.L. van Otterlo, S.F. van Vuuren, C.B. de Koning, Bioorg. Med. Chem. Lett. 19, 4948 (2009)
N.D. Kumar, M. Kumar, S. Sundaree, O.E. Johnson, K. Shah, Eur. J. Med. Chem. 45, 1244 (2010)
Y.Y. Li, H.S. Wu, L. Tang, C.R. Feng, H.Y. Yu, Y.S. Yang, B.J. Yang, Pharmacol. Res. 56, 335 (2007)
S. Kumar, H. Kaur, K.K. Saxena, M. Sharma, P. Vishwakarma, A. Kumar, Indian J. Chem. 49, 1398 (2010)
P. Rani, V.K. Srivastava, A. Kumar, Eur. J. Med. Chem. 39, 449 (2004)
H. Abdel-Gawad, H.A. Mohamed, K.M. Dawood, F.A. Badria, Chem. Pharm. Bull. 58, 1529 (2010)
W. Hu, Z. Guo, X. Yi, C. Guo, F. Chu, G. Cheng, Bioorg. Med. Chem. 11, 5539 (2003)
N.D. Kumar, M. Kumar, B. Noel, K. Shah, Eur. J. Med. Chem. 55, 432 (2012)
D.B. England, G. Merey, A. Padwa, Org. Lett. 19, 3805 (2007)
X. Ban, Y. Pan, Y.F. Lin, S.Q. Wang, Y.F. Du, K. Zhao, Org. Biomol. Chem. 10, 3606 (2012)
J.T. Kuethe, A. Wong, I.W. Davies, Org. Lett. 5, 3975 (2003)
G.W. Gribble, J. Chem. Soc. Perkin Trans. 1, 1045 (2000)
Z. Liang, J. Zhao, Y.J. Zhang, Org. Chem. 75, 170 (2010)
J.E. DeLorbe, S.Y. Jabri, S.M. Mennen, L.E. Overman, F.-L. Zhang, J. Am. Chem. Soc. 133, 6549 (2011)
T.A. Fenoradosoa, G. Ali, C. Delattre, C. Laroche, E. Petit, A. Wadouachi, P. Michaud, J. Appl. Phtcol. 22, 131 (2010)
P. Vauchel, R. Kaas, A. Arhaliass, R. Baron, J. Legrand, Food Bioprocess Tech. 1, 297 (2008)
M. Glicksman, Adv. Food Res. 11, 109 (1963)
S. Ilkhanizadeh, J. Khalafy, M.G. Dekamin, Int. J. Biol. Macromol. 140, 605 (2019)
B. Eftekhari-Sis, M. Zirak, A. Akbari, Chem. Rev. 113, 2958 (2013)
J. Khalafy, N. Etivand, S. Dilmaghani, M. Ezzati, A. Poursattar Marjani, Tetrahedron Lett. 55, 3781 (2014)
M. Ezzati, J. Khalafy, A. Poursattar Marjani, R.H. Prager, Tetrahedron 73, 6587 (2017)
A. Poursattar Marjani, J. Khalafy, F. Majidi Arlan, E. Eyni, ARKIVOC v, 1 (2019)
N. Etivand, J. Khalafy, A. Poursattar Marjani, Res. Chem. Intermed. 45, 3379 (2019)
J. Khalafy, N. Etivand, A. Poursattar Marjani, N. Khalillou, J. Heterocycl. Chem. 56, 1857 (2019)
A. Poursattar Marjani, J. Khalafy, S. Akbarzadeh, Green Process Synth. 8, 533 (2019)
A. Nouri, A. Poursattar Marjani, J. Khalafy, J. Heterocycl. Chem. 56, 2912 (2019)
A. Poursattar Marjani, J. Khalafy, P. Eslamipour, M. Ahmadi Sabegh, Iran J. Chem. Chem. Eng. 38, 51 (2019)
M. Aslanpanjeh, A. Poursattar Marjani, J. Khalafy, N. Etivand, Res. Chem. Intermed. 46, 165 (2020)
Acknowledgements
The authors are appreciatively thankful for the support provided by the Urmia University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nouri, A., Marjani, A.P., Khalafy, J. et al. A new and facile synthesis of 3-(2-aryl-6-nitro-1H-indol-3-yl)quinoline-2,4(1H,3H)-diones by sodium alginate as biopolymeric catalyst. Res Chem Intermed 46, 3025–3036 (2020). https://doi.org/10.1007/s11164-020-04129-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04129-4