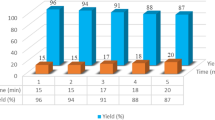
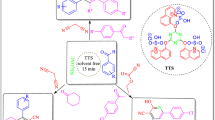
Abstract
In the present work, 3,3′-(butane-1,4-diyl)bis(1-methyl-1H-imidazol-3-ium) dichloride ([C4(MIm)2]·2Cl) and 3,3′-(butane-1,4-diyl)bis(1-methyl-1H-imidazol-3-ium) hydrogen sulfate ([C4(MIm)2]·2HSO4) were prepared via adequate, solvent-free methods and characterized using FT-IR, 1H NMR, 13C NMR, and potentiometric titration techniques. These reagents were investigated in the synthesis of 5-arylidene (thio)barbituric acids, 2-arylidene malononitriles, 4H-pyrans, and pyrano[2,3-d] pyrimidineones, and their catalytic activities were compared in the promotion of these reactions. Based on the obtained results, we found that [C4(MIm)2]·2Cl showed more catalytic efficiency where a basic or weak acidic media is needed. In contrast, [C4(MIm)2]·2HSO4 is a powerful catalyst in reactions needing acidic catalysts to enhance the reaction rate. Using these reagents, the products were formed under mild and eco-friendly conditions in excellent yields during short reaction times without needing column chromatography for work-up.











Similar content being viewed by others
References
D. Kuang, P. Wang, S. Ito, S.M. Zakeeruddin, M. Gratzel, J. Am. Chem. Soc. 128, 7732 (2006)
M. Ue, M. Takeda, T. Takahashi, M. Takehara, Electrochem. Solid State Lett. 5, A119 (2002)
A. Noda, M.A.B.H. Susan, K. Kudo, S. Mitsushima, K. Hayamizu, M. Watanabe, J. Phys. Chem. B 107, 4024 (2003)
A. Marciniak, Fluid Phase Equilib. 294, 213 (2010)
P. Bhanuprakash, N.V.V. Jyothi, C. Narasimharao, M. Raveendra, K. Sivakumar, J. Chem. Thermodyn. 112, 113 (2018)
B. Wu, R. G. Reddy, R. D. Rogers, Proceedings of Solar Forum 2001. Solar Energy: The Power to Choose, April 22–35 (Washington, DC, 2001)
K. Ito, N. Nishina, H. Ohno, Electrochim. Acta 45, 1295 (2000)
J.F. Oskooie, S.I. Lall-Ramnarine, R. Raju, A. Scumpia, S. Bellevue, R. Ragbir, R. Engel, Radiat. Phys. Chem. 72, 99 (2005)
J.L. Anderson, R. Ding, A. Ellern, D.W. Armstrong, J. Am. Chem. Soc. 127, 593 (2005)
X. Han, D.W. Armstrong, Org. Lett. 7, 4205 (2005)
H. Nakajima, H. Ohno, Polymer 46, 11499 (2005)
Q. Liu, F. van Rantwijk, R.A. Sheldon, J. Chem. Technol. Biotechnol. 81, 401 (2006)
Z. Zhang, L. Yang, S. Luo, M. Tian, K. Tachibana, K. Kamijima, J. Power Sources 167, 217 (2007)
Q.Q. Baltazar, J. Chandawalla, K. Sawyer, J.L. Anderson, Colloids Surf. A Physicochem. Eng. Asp. 302, 150 (2007)
T. Payagala, J. Huang, Z.S. Breitbach, P.S. Sharma, D.W. Armstrong, Chem. Mater. 19, 5848 (2007)
Z. Zhang, H. Zhou, L. Yang, K. Tachibana, K. Kamijima, J. Xu, Electrochim. Acta 53, 4833 (2008)
N. Daneshvar, M. Nasiri, M. Shirzad, M.S.N. Langarudi, F. Shirini, H. Tajik, New J. Chem. 42, 9744 (2018)
A. Chinnappan, H. Kim, Chem. Eng. J. 187, 283 (2012)
H. Jadhav, H. Kim, Tetrahedron Lett. 53, 5338 (2012)
M. Messali, Z. Moussa, A.Y. Alzahrani, M.Y. El-Naggar, A.S. ElDouhaibi, Z.M.A. Judeh, B. Hammouti, Chemosphere 91, 1627 (2013)
C. Ranu, S. Banerjee, Org. Lett. 7, 3049 (2005)
T. Payagala, J. Huang, S.B. Zachary, S.S. Pritesh, W.A. Daniel, Chem. Mater. 19, 5848 (2007)
J. Davoll, J. Clarke, E.F. Eislager, J. Med. Chem. 15, 837 (1972)
A.D. Broom, J.L. Shim, G.L. Anderson, J. Org. Chem. 41, 1095 (1976)
E.M. Griva, S. Lee, C.W. Siyal, D.S. Duch, C.A. Nichol, J. Med. Chem. 23, 327 (1980)
D. Heber, C. Heers, U. Ravens, Pharmazie 48, 537 (1993)
M.M. Ghorab, A.Y. Hassan, Phosphorus Sulfur Silicon 141, 251 (1998)
K. Kantharaju, P.B. Hiremath, Int. J. Eng. Technol. Sci. Res. 4, 808 (2017)
P.S. Sharma, T. Payagala, E. Wanigasekara, A.B. Wijeratne, J. Huang, D.W. Armstrong, Chem. Mater. 20, 4182 (2008)
J. Zamocka, E. Misikova, J. Durinda, Pharmazie 46, 610 (1991)
H.M. Aly, M.M. Kamal, Eur. J. Med. Chem. 47, 18 (2012)
N.R. Kamdar, D.D. Haveliwala, P.T. Mistry, S.K. Patel, Eur. J. Med. Chem. 45, 5056 (2010)
A.H. Shamroukh, M.E.A. Zaki, E.M.H. Morsy, F.M. Abdel-Motti, F.M.E. Abdel-Meguid, Arch. Pharm. 340, 36 (2007)
D.R. Mohamed, M.M. Abdel Halim, Y.A. Khadrawy, G.A. El Megeed, O.M.E. Abdel-Salam, Steroids 77, 1469 (2012)
E.C. Witte, P. Neubert, A. Roesch, Ger. Offen. DE, 3427985 (1986)
J.L. Wang, D. Liu, Z.J. Zhang, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z. Huang, Proc. Natl. Acad. Sci. U.S.A. 97, 7124 (2000)
N.M. Sabry, H.M. Mohamed, E. Khattab, S.S. Motlaq, A.M. El-Agrody, Eur. J. Med. Chem. 46, 765 (2011)
N.A. Al-Masoudi, H.H. Mohammed, A.M. Hamdy, O.A. Akrawi, N. Eleya, A. Spannenberg, C. Pannecouque, P.Z. Langer, Naturforsch. 68b, 229 (2013)
Sh Uzzaman, A. Mahmood, Y. Khan, A. Sohail, J. Photochem. Photobiol. B Biol. 129, 36 (2013)
D. Zhao, M. Liu, J. Zhang, J. Li, P. Ren, Chem. Eng. J. 221, 99 (2013)
A.H. Tamboli, H.A. Bandal, H. Kim, Chem. Eng. J. 306, 826 (2016)
N. Seyyedi, F. Shirini, M.S.N. Langarudi, RSC Adv. 6, 44630 (2016)
S. Jashnani, M. Seddighi, M.S.N. Langarudi, F. Shirini, ChemistrySelect 3, 11585 (2018)
F. Shirini, M.S.N. Langarudi, N. Daneshvar, M. Mashhadinezhad, N. Nabinia, J. Mol. Liq. 243, 302 (2017)
F. Shirini, M.S.N. Langarudi, N. Daneshvar, N. Jamasbi, M. Irankhah-Khanghah, J. Mol. Struct. 1161, 366 (2018)
N. Jamasbi, M. Irankhah-Khanghah, F. Shirini, H. Tajik, M.S.N. Langarudi, New J. Chem. 42, 9016 (2018)
J. Li, H. Dai, D. Liu, T. Li, Synth. Commun. 36, 789 (2006)
C. Wang, Synth. Commun. 35, 2759 (2005)
L.S. Gadekar, M.K. Lande, Org. Chem. Indian J. 8, 386 (2012)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
S. Wang, Z. Ren, W. Cao, W. Tong, Synth. Commun. 31, 673 (2001)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625 (2004)
S. Gao, C.H. Tsai, C. Tseng, C. Yao, Tetrahedron 64, 9143 (2008)
I. López, J.L. Bravo, M. Caraballo, J.L. Barneto, G. Silvero, Tetrahedron Lett. 52, 3339 (2011)
H.A. Oskooie, M.M. Heravi, N. Karimi, M.E. Zadeh, Synth. Commun. 41, 436 (2011)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
H. Zhi, C. Lu, Q. Zhang, J. Luo, Chem. Commun. 2878 (2009)
A. Hasaninejad, M. Shekouhy, N. Golzar, A. Zare, M.M. Doroodmand, Appl. Catal. A 402, 11 (2011)
F. Shirini, O. Goli-Jolodar, M. Akbari, M. Seddighi, Res. Chem. Intermed. 42, 4733 (2016)
J.M. Khurana, K. Vij, Catal. Lett. 138, 104 (2010)
M. Bararjanian, S. Balalaie, B. Movassagh, A.M. Amani, J. Iran. Chem. Soc. 6, 436 (2009)
G.M. Ziarani, S. Faramarzi, S. Asadi, A. Badiei, R. Bazl, M. Amanlou, Daru J. Pharm. Sci. 21, 3 (2013)
A. Mobinikhaledi, M.A. Bodaghi Fard, Acta Chim. Slov. 57, 931 (2010)
S. Mashkouri, M.R. Naimi-Jamal, Molecules 14, 474 (2009)
M.M. Heravi, A. Ghods, K. Bakhtiari, F. Derikvand, Synth. Commun. 40, 1927 (2010)
F. Shirini, N. Daneshvar, M.S.N. Langarudi, R. Karimi-Chayjani, Bioorg. Chem. 77, 68 (2018)
Y. Cai, Y. Peng, G. Song, Catal. Lett. 109, 61 (2006)
XZh Lian, Y. Huang, Y.Q. Li, W.J. Zheng, Monatsh. Chem. 139, 129 (2008)
S. Gurumurthi, V. Sundari, R. Valliappan, J. Chem. 6, S466 (2009)
E. Sheikhhosseini, D. Ghazanfari, V. Nezamabadi, Iran J. Catal. 3, 197 (2013)
F. Shirini, M.S.N. Langarudi, N. Daneshvar, J. Mol. Liq. 234, 268 (2017)
J. Azizian, A. Shameli, S. Balalaie, M.M. Ghanbari, S. Zomorodbakhsh, M. Entezari, S. Bagheri, G. Fakhrpour, Orient. J. Chem. 28, 327 (2012)
J. Yu, H.Q. Wang, Synth. Commun. 35, 3133 (2005)
Acknowledgements
Facilitation of the studies by the University of Guilan Research Council is appreciably acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharifi, Z., Daneshvar, N., Langarudi, M.S.N. et al. Comparison of the efficiency of two imidazole-based dicationic ionic liquids as the catalysts in the synthesis of pyran derivatives and Knoevenagel condensations. Res Chem Intermed 45, 4941–4958 (2019). https://doi.org/10.1007/s11164-019-03874-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03874-5