Abstract
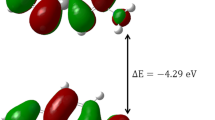
The compounds 4,8,9,10-tetraaryl-1,3-diazaadamantan-6-ones (a–e) were synthesized and characterized by FT-IR, 1H and 13C NMR spectra, and the spectral data have been theoretically analyzed by the DFT method. The electronic properties including a highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO) and related parameters were calculated with B3LYP/6-311 G (d, p) basis set. The observed HOMO and LUMO mappings describe the different charge transfer possibilities within the molecule. Besides, the reactive properties of the molecules have been addressed based on the frontier molecular orbital analysis. The Molecular Electrostatic Potential and Fukui function analysis reveal the sites for electrophilic attack and nucleophilic reactions in the molecule. The intramolecular contacts have been interpreted using Natural Bond Orbital analysis and the thermodynamic properties also presented. The molecular docking study has been executed to study the binding interactions of the synthesized compounds with H1N1 swine virus-M2 proton channel and COX-2 protein.
Graphical abstract











Similar content being viewed by others
References
W.L. Davies, R.R. Grunnert, R.F. Haff, J.W. McGahen, E.M. Neumeyer, M. Paulshock, J.C. Watts, T.R. Wood, E.C. Hermann, C.E. Hoffmann, Science 144, 862 (1964)
Y. Togo, R.B. Hornick, A.T. Dawkins, J. Am. Med. Assoc. 203, 1089 (1968)
F.G. Hayden, J.M.I. Gwaltney, C.R.L. Van, K.F. Adams, B. Giordani, Antimicrob. Agents Chemother. 19, 226 (1981)
S. Rabinovich, J.T. Baldini, R. Bannister, Am. J. Med. Sci. 257, 328 (1969)
J. Balzarini, B. Orzeszko, J.K. Maurin, A. Orzeszko, Eur. J. Med. Chem. 42, 993 (2007)
J. Balzarini, B. Orzeszko-Krzesińska, J.K. Maurin, A. Orzeszko, Eur. J. Med. Chem. 44, 303 (2009)
M.A. Al-Omar, E.S. Al-Abdullah, I.A. Shehata, E.E. Habib, T.M. Ibrahim, A.A. El-Emam, Molecules 15, 2526 (2010)
O. Kouatly, A. Geronikaki, C. Kamoutsis, D. Hadjipavlou-Litina, P. Eleftheriou, Eur. J. Med. Chem. 44, 119 (2009)
A.A. Kadi, E.S. Al-Abdullah, I.A. Shehata, E.E. Habib, T.M. Ibrahim, A.A. El-Emam, Eur. J. Med. Chem. 45, 5006 (2010)
G. Zoidis, I. Papanastasiou, I. Dotsikas, A. Sandoval, R.G. Dos Santos, Z. Papdopoolou-Daifoti, A. Vamvakides, N. Kolocouris, R. Felix, Bioorg. Med. Chem. 13, 2791 (2005)
A. Baxter, J. Bent, K. Bowers, M. Braddock, S. Brough, M. Fagura, M. Lawson, T. McInally, M. Mortimore, M. Robertson, R. Weaver, P. Webborn, Bioorg. Med. Chem. Lett. 13, 4047 (2003)
J.C.E. Owen, P.S. Whitton, Brain Res. 1117, 206 (2006)
E.B. Villhauer, J.A. Brinkman, G.B. Naderi, B.F. Burkey, B.E. Dunning, K. Prasad, B.L. Mangold, M.E. Russell, T.E. Hughes, J. Med. Chem. 46, 2774 (2003)
D.J. Augeri, J.A. Robl, D.A. Betebenner, D.R. Magnin, A. Khanna, J.G. Robertson, A. Wang, L.M. Simpkins, P. Taunk, Q. Huang, S. Han, B. Abboa-Offei, M. Cap, L. Xin, L. Tao, E. Tozzo, G.E. Welzel, D.M. Egan, J. Marcinkeviciene, S.Y. Chang, S.A. Biller, M.S. Kirby, R.A. Parker, L.G. Hamann, J. Med. Chem. 48, 5025 (2005)
M.S. Scherman, E.J. North, V. Jones, T.N. Hess, A.E. Grzegorzewicz, T. Kasagami, I.H. Kim, O. Merzlikin, A.J. Lenaerts, R.E. Lee, M. Jackson, C. Morisseau, M.R. McNeil, Bioorg. Med. Chem. 20, 3255 (2012)
A.A. El-Emam, A.M.S. Al-Tamimi, M.A. Al-Omar, K.A. Alrashood, E.E. Habib, Eur. J. Med. Chem. 68, 96 (2013)
E.J. North, M.S. Scherman, D.F. Bruhn, J.S. Scarborough, M.M. Maddox, V. Jones, A. Grzegorzewicz, L. Yang, T. Hess, C. Morisseau, M. Jackson, M.R. McNeil, R.E. Lee, Bioorg. Med. Chem. 21, 2587 (2013)
E.S. Al-Abdullah, H.M. Al-Tuwaijri, H.M. Hassan, M.A. Al-Alshaikh, E.E. Habib, A.A. El-Emam, Molecules 20, 8125 (2015)
L.H. Al-Wahaibi, H.M. Hassan, A.M. Abo-Kamar, H.A. Ghabbour, A.A. El-Emam, Molecules 22, 710 (2017)
G.L. Balaji, S. Sarveswari, V. Vijayakumar, Res. Chem. Intermed. 41, 6765 (2015)
S. Sivasubramanian, M. Sundaravadivelu, N. Arumugam, Org. Prep. Proced. Int. 22, 645 (1990)
V. Vijayakumar, M. Sundaravadivelu, S. Perumal, Magn. Reson. Chem. 39, 101 (2001)
J.F. Wang, D.Q. Wei, K.C. Chou, Biochem. Biophys. Res. Commun. 388, 413 (2009)
K.M. Amin, M.M. Kamel, M.M. Anwar, M. Khedr, Y.M. Syam, Eur. J. Med. Chem. 45, 2117 (2010)
G.M. Morris, D.S. Goodsell, R.S. Halliday, R. Huey, W.E. Hart, R.K. Belew, A.J. Olson, J. Comput. Chem. 19, 1639 (1998)
M.J. Frisch, J.A. Pople, J.S. Binkley, J. Chem. Phys. 80, 3265 (1984)
R. Ditchfield, Mol. Phys. 27, 789 (1974)
C.Y. Panicker, H.T. Varghese, P.S. Nayak, B. Narayana, B.K. Sarojini, H.K. Fun, J.A. War, S.K. Srivastava, C.V. Alsenoy, Spectrochim Acta A 148, 18 (2015)
R.G. Parr, R.G. Pearson, J. Am. Chem. Soc. 105, 7512 (1983)
R.G. Pearson, Proc. Natl. Acad. Sci. USA 83, 8440 (1986)
R.G. Parr, L.V. Szentpaly, S. Liu, J. Am. Chem. Soc. 121, 1922 (1999)
Z. Zhou, R.G. Parr, J. Am. Chem. Soc. 112, 5720 (1990)
D. Mahadevan, S. Periandy, M. Karabacak, S. Ramalingam, N. Puviarasan, Spectrochim. Acta A 86, 139 (2012)
J. Aihara, Theor. Chem. Acc. 102, 134 (1999)
J. Aihara, J. Phys. Chem. A 103, 7487 (1999)
T.K. Kuruvilla, J.C. Prasana, S. Muthu, J. George, S. AnnMathew, Spectrochim. Acta A 188, 382 (2018)
F.A.M. Al-Omary, A. Raj, K. Raju, C. Yohannan Panicker, N.G. Haress, A.A. El-Emam, M.B. El-Ashmawy, A.A. Al-Saadi, C.V. Alsenoy, J. Ahmad War, Spectrochim. Acta A 136, 520 (2015)
E. Scrocco, J. Tomasi, Adv. Quantum Chem. 11, 115 (1979)
E.J. Luque, J.M. Lopez, M. Orozco, Theor. Chem. Acc. 103, 343 (2000)
T. Karthick, P. Tandon, J. Mol. Model. 22, 142 (2016)
J. Lu, W.R. Kobertz, C. Deutsch, J. Mol. Biol. 371, 1378 (2007)
N. Uludağ, G. Serdaroğlu, A. Yinanc, J. Mol. Struct. 1161, 152 (2018)
A. Esme, S.G. Sagdinç, Spectrochim. Acta A 188, 443 (2018)
F. Weinhold, C.R. Landis, E.D. Glendening, Int. Rev. Phys. Chem. 35, 399 (2016)
A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. 88, 899 (1988)
M. Raja, R. Raj Muhamed, S. Muthu, M. Suresh, J. Mol. Struct. 1141, 284 (2017)
A.O. Zacharias, A. Varghese, K.B. Akshaya, M.S. Savitha, L. George, J. Mol. Struct. 1158, 1 (2018)
C. Morell, A. Grand, A. Toro-Labbe, J. Phys. Chem. A 109, 205 (2005)
S. Sevanthi, S. Muthu, M. Raja, J. Mol. Struct. 1173, 251 (2018)
Acknowledgements
The authors thank the UGC, New Delhi for Major Research Project (Grant No. 42-358/2013 (SR)) & UGC-Special Assistance Program (SAP). The authors gratefully acknowledge the DST- FIST NMR facility of Department of Chemistry, Gandhigram Rural Institute-Deemed to be University for recording NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vengatesh, G., Sundaravadivelu, M. Quantum chemical, experimental, theoretical spectral (FT-IR and NMR) studies and molecular docking investigation of 4,8,9,10-tetraaryl-1,3-diazaadamantan-6-ones. Res Chem Intermed 45, 4395–4415 (2019). https://doi.org/10.1007/s11164-019-03838-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03838-9