Abstract
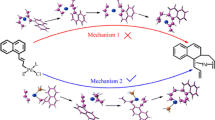
A novel, efficient, one-pot, catalyst-free grinding procedure for synthesis of 6-amino-3-methyl-4-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile is reported. The condensation of substituted benzaldehydes, 3-amino-5-methylpyrazole, and malononitrile according to a three-component reaction was investigated using density functional theory (DFT) at B3LYP/6-311G level to explore the reaction mechanism. All the routes were studied, the structure of the intermediates was optimized, and all the respective transition states were found. The results of the calculations show that the proposed mechanism relies on four intermediates.






Similar content being viewed by others
References
A.M. Li, Y. Ouyang, Z.Y. Wang, Y.Y. Cao, X.Y. Liu, L. Ran, C. Li, L. Li, L. Zhang, K. Qiao, J. Med. Chem. 56, 3593 (2013)
A.H. Shamroukh, A.E. Rashad, H.H. Sayed, J. Phosphorus Sulfur Silicon Rel. Elem. 180, 2347 (2005)
L. Commeiras, S.C. Woodcock, J.E. Baldwin, R.M. Adlington, A.R. Cowley, P. Wilkinson, Tetrahedron 60, 933 (2004)
A. Cappelli, C. Nannicini, A. Gallelli, G. Giuliani, S. Valenti, G.P. Mohr, M. Anzini, L. Mennuni, F. Ferrari, G. Caselli, A. Giordani, W. Pereis, F. Makovec, G. Giorgi, S. Vomero, J. Med. Chem. 51, 2137 (2008)
R. Lin, P.J. Connolly, Y. Lu, G. Chin, S. Li, Y. Yu, S. Huang, X. Li, S.L. Emanuel, S.A. Middleton, R.H. Gruninger, M. Adams, A.R. Fuentes-Pesquera, L.M. Greenberger, J. Bioorg. Med. Chem. Lett. 17, 4557 (2007)
H. de Mello, A. Echevarria, A.M. Bernardino, M. CantoCavalheiro, L.L. Leon, J. Med. Chem. 47, 5427 (2004)
F. Manetti, S. Schenone, F. Bondavalli, C. Brullo, O. Bruno, A. Ranise, L. Mosti, G. Menozzi, P. Fossa, M.L. Trincavelli, C. Martini, A. Martinelli, C. Tintori, M. Botta, J. Med. Chem. 48, 7172 (2005)
B.A. Johns, K.S. Gudmundsson, E.M. Turner, S.H. Allen, V.A. Samano, J.A. Ray, G.A. Freeman, F.L. Boyd, C.J. Sexton, D.W. Selleseth, K.L. Creech, K.R. Moniri, J. Bioorg. Med. Chem. 13, 2397 (2005)
K.S. Gudmundsson, B.A. Johns, Z. Wang, E.M. Turner, S.H. Allen, G.A. Freeman, F.L.B. Jr, C.J. Sexton, D.W. Selleseth, K.R. Monirib, K.L. Creech, J. Bioorg. Med. Chem. 13, 5346 (2005)
F.E. Goda, A.A.M. Abdel-Aziz, O.A. Attef, J. Bioorg. Med. Chem. Lett. 12, 1845 (2004)
N.M. Parekh, K.C. Maheria, J. Res. Chem. Intermed. 38, 885 (2012)
L. Zare, N.O. Mahmoodi, A. Yahyazadeh, M. Mamaghani, Syn. Commun. 41, 2323 (2011)
P.A. Wender, J. Nat. Prod. Rep. 31, 433 (2014)
A.Z. Halimehjani, I.N. Namboothiri, S.E. Hooshmand, RSC Adv. 4, 48022 (2014)
A.Z. Halimehjani, I.N. Namboothiri, S.E. Hooshmand, RSC Adv. 4, 51794 (2014)
V. Estévez, M. Villacampa, J.C. Menéndez, J. Chem. Soc. Rev. 43, 4633 (2014)
A. Domling, W. Wang, K. Wang, J. Chem. Rev. 112, 3083 (2012)
M. Nikpassand, L. Zare Fekri, M. Nabatzadeh, Comb. Chem. High Throughput Screen. 20, 533 (2017)
L. Zare Fekri, M. Nikpassand, J. Chil. Chem. Soc. 57, 1415 (2012)
M. Nikpassand, L. ZareFekri, S. Sanagou, Dyes Pigm. 136, 140 (2017)
H. Taherkhorsand, M. Nikpassand, Comb. Chem. High Throughput Screen. 21, 65 (2018)
L. Zare Fekri, M. Nikpassand, M. Goldoost, Russ. J. Gen. Chem. 83, 2352 (2013)
L. Zare Fekri, M. Nikpassand, Russ. J. Gen. Chem. 83, 2395 (2013)
M. Nikpassand, L. Zare Fekri, K. Hematinezhad, J. Polycycl. Arom. Comp. in press (2018)
M.J. Frisch, et al., GAUSSIAN09, Gaussian, Inc., Revision B. 05, Pittsburgh PA (2009)
J.G. Ma, J.M. Zhang, H.H. Jiang, W.Y. Ma, J.H. Zhou, Chin. Chem. Lett. 19, 375 (2008)
S. Karmakar, A. Datta, J. Org. Chem. 82, 1558 (2017)
S. Karmakar, A. Datta, J. Phys. Chem. B 121, 7621 (2017)
K. Bhattacharyya, S. Karmakar, A. Datta, Phys. Chem. Chem. Phys. 19, 22482 (2017)
Acknowledgements
Financial support from the Research Council of Islamic Azad University, Rasht Branch is sincerely acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikpassand, M., Fekri, L.Z. & Rahro, P.N. Catalyst-free grinding method: a new avenue for synthesis of 6-amino-3-methyl-4-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile and DFT studies on the mechanistic pathway of this category of compounds. Res Chem Intermed 45, 1707–1719 (2019). https://doi.org/10.1007/s11164-018-3701-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3701-9