Abstract
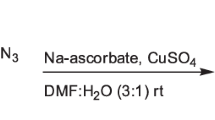
A series of 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives (8a–p) have been synthesized. The structure of the newly synthesized compounds was determined by spectral analysis. The title compounds were screened for their preliminary antitubercular activity against Mycobacterium tuberculosis H37Ra (MTB, ATCC 25177) and Mycobacterium bovis BCG (BCG, ATCC 35743). Further, the synthesized compounds were screened for antimicrobial activity against standard Gram-negative bacteria Escherichia coli (NCIM 2576) and Pseudomonas flurescence (NCIM 2059) and Gram-positive bacteria Staphylococcus aureus (NCIM 2602) and Bacillus subtilis (NCIM 2162). Among all the synthesized compounds, 8a–c, f–h, m exhibited good activity against dormant M. bovis BCG strain. Compounds 8h, j exhibited good activity against all tested bacterial strains. All active compounds were screened for cytotoxicity and found inactive. Their high potency and promising antimycobacterial activity suggest that these compounds could serve as good leads for further optimization and development.


Similar content being viewed by others
References
World Health Organization, Tuberculosis Fact Sheet (2016), http://www.who.int/tb/publications/global_report/en/
Q. Wang, F. Song, X. Xiao, P. Huang, L. Li, A. Monte, W.M. Abdel-Mageed, J. Wang, H. Guo, W. He, F. Xie, H. Dai, M. Liu, C. Chen, H. Xu, M. Liu, A.M. Piggott, X. Liu, R.J. Capon, L. Zhang, Angew. Chem. Int. Ed. 52, 1231–1234 (2013)
R. Ramesh, R.D. Shingare, V. Kumar, A. Anand, B. Swetha, S. Veeraraghavan, S. Viswanadha, R. Ummanni, R. Gokhale, D.S. Reddy, Eur. J. Med. Chem. 122, 723–730 (2016)
V.U. Jeankumar, S.R. Rudraraju, R. Vats, R. Janupally, S. Saxena, P. Yogeeswari, D. Sriram, Eur. J. Med. Chem. 122, 216–231 (2016)
M.D. Chen, S.J. Lu, G.P. Yuag, S.Y. Yang, X.L. Du, Heterocycl. Commun. 6, 421–426 (2000)
B.S. Holla, M. Mahalinga, M.S. Karthikeyen, B. Poojary, P.M. Akberali, N.S. Kumari, Eur. J. Med. Chem. 40, 1173–1178 (2005)
H.N. Hafez, H.A. Abbas, A.R. El-Gazzar, Acta Pharm. 58, 359–378 (2008)
A. Passannanti, P. Diana, P. Barraja, F. Mingooia, A. Lauria, G. Cirrincine, Heterocycles 48, 1229–1235 (1998)
L.P. Guan, Q.H. Jin, G.R. Tian, K.Y. Chai, Z.S. Quan, J. Pharm. Sci. 10, 254–262 (2007)
S. Manfredini, C.B. Vicentini, M. Manfrini, N. Bianchi, C. Rustigliano, C. Mischiati, R. Gambari, Bioorg. Med. Chem. 8, 2343–2346 (2000)
V. Dmitry, A.V. Demchuk, N.B. Samet, V.I. Chernysheva, G.A. Ushkarov, L.D. Stashina, M.M. Konyushkin, S.I. Raihstat, A.A. Firgang, M.P. Philchenkov, L.M. Zavelevich, V.F. Kuiava, D.Y. Chekhun, A.S. Blokhin, M.N. Kiselyov, V.V. Semenova, Bioorg. Med. Chem. 22, 738–755 (2014)
R. Gujjar, A. Marwaha, J. White, L. White, S. Creason, D.M. Shackleford, J. Baldwin, W.N. Charman, F.S. Buckner, S. Charman, P.K. Rathod, M.A. Phillips, J. Med. Chem. 52, 1864–1872 (2009)
B.A. Johns, J.G. Weatherhead, S.H. Allen, J.B. Thompson, E.P. Garvey, S.A. Foster, Bioorg. Med. Chem. Lett. 19, 1802–1806 (2009)
S.C. Beasley, N. Cooper, L. Gowers, J.P. Gregory, A.A.F. Haughan, P.G. Hellewell, D. Macar, J. Miotla, J.G. Montana, T. Morgan, R. Taylor, K.A. Runcie, B. Tuladhar, J.B.H. Warneck, Bioorg. Med. Chem. Lett. 8, 2629–2634 (1998)
C.E. De, Clin. Microbiol. Rev. 10, 674–693 (1997)
T. Weide, S.A. Saldanha, D. Minond, T.P. Spicer, J.R. Fotsing, M. Spaargaren, J. Frere, C. Bebrone, K.B. Sharpless, P.S. Hodder, V.V. Fokin, A.C.S. Med, Chem. Lett. 1, 150–154 (2010)
G. Heubach, B. Sachse, H. Buerstell, Ger. Offen 2, 760–826 (1979)
B. Chaia, X. Qianb, S. Caoa, H. Liua, G. Song, ARKIVOC ii, 141-145, (2003)
A.R. Bhat, G.V. Bhat, G.G. Shenoy, J. Pharm. Pharmacol. 53, 267–272 (2001)
N.B. Patel, I.H. Khan, S.D. Rajani, Eur. J. Med. Chem. 45, 4293–4299 (2010)
G.R. Jadhav, M.U. Shaikh, R.P. Kale, M.R. Shiradkar, C.H. Gill, Eur. J. Med. Chem. 44, 2930–2935 (2009)
H. Foks, M. Janowiec, Z. Zwolska, E. Augustynowicz-Kopeć, Phosphorus Sulfur Silicon Relat. Elem. 180, 537–543 (2005)
D. Castagnolo, M. Radi, F. Dessi, F. Manetti, M. Saddi, R. Meleddu, A.D. Logu, M. Botta, Bioorg. Med. Chem. Lett. 19, 2203–2205 (2009)
M. Shiradkar, S. Kumar, V. Dasari, S. Tatikonda, K.C. Akula, R. Shah, Eur. J. Med. Chem. 42, 807–816 (2007)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, A.T. Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911–3922 (2012)
M.H. Shaikh, D.D. Subhedar, L. Nawale, D. Sarkar, F.A. Kalam Khan, J.N. Sangshetti, B.B. Shingate, Med. Chem. Commun 6, 1104–1116 (2015)
P. Shanmugavelan, S. Nagarajan, M. Sathishkumar, A. Ponnuswamy, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. Lett. 21, 7273–7276 (2011)
N. Boechat, V.F. Ferreira, S.B. Ferreira, M.L.G. Ferreira, F.C. da Silva, M.M. Bastos, M.S. Costa, M.C.S. Lourenço, A.C. Pinto, A.U. Krettli, A.C. Aguiar, B.M. Teixeira, N.V. da Silva, P.R.C. Martins, F.A.F.M. Bezerra, A.L.S. Camilo, G.P. da Silva, C.C.P. Costa, J. Med. Chem. 54, 5988–5999 (2011)
G.R. Labadie, A. de la Iglesia, H.R. Morbidoni, Mol. Divers. 15, 1017–1024 (2011)
C. Gill, G. Jadhav, M. Shaikh, R. Kale, A. Ghawalkar, D. Nagargoje, M. Shiradkar, Bioorg. Med. Chem. Lett. 18, 6244–6247 (2008)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, T. Anjana Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911–3922 (2012)
L. Chen, D.J. Wilson, Y. Xu, C.C. Aldrich, K. Felczak, Y.Y. Sham, K.W. Pankiewicz, J. Med. Chem. 53, 4768–4778 (2010)
R.S. Upadhayaya, G.M. Kulkarni, N.R. Vasireddy, J.K. Vandavasi, S.S. Dixit, V. Sharma, J. Chattopadhyaya, Bioorg. Med. Chem. 17, 4681–4692 (2009)
C. Menendez, S. Gau, C. Lherbet, F. Rodriguez, C. Inard, M.R. Pasca, M. Baltas, Eur. J. Med. Chem. 46, 5524–5531 (2011)
S.P. Pardeshi, S.S. Patil, V.D. Bobade, Bioorg. Med. Chem. Lett. 21, 6559–6562 (2011)
T. Tomasic, S. Katsamakas, Z. Hodnik, J. Ilas, M. Brvar, T. Solmajer, S. Montalvao, P. Tammela, M. Banjanac, G. Ergovic, M. Anderluh, L.P. Masic, D. Kikelj, J. Med. Chem. 58, 5501–5521 (2015)
Z.Y. Liu, Y.M. Wang, Z.R. Li, J.D. Jiang, D.W. Boykin, Bioorg. Med. Chem. Lett. 19, 5661–5664 (2009)
S.A.F. Rostom, I.M. El-Ashmawy, H.A. Abd El Razik, M.H. Badr, H.M.A. Ashour, Bioorg. Med. Chem. Lett. 17, 882–895 (2009)
D. Sampson, X.Y. Zhu, S.V.K. Eyunni, J.R. Etukala, E. Ofori, B. Bricker, N.S. Lamango, V. Setola, B. Roth, S.Y. Ablordeppey, Bioorg. Med. Chem. Lett. 22, 3105–3114 (2014)
G.S. Inamdar, A.N. Pandya, H.M. Thakar, V. Sudarsanam, S. Kachler, S. Moro, D. Sabbadin, K.N. Klotz, K.K. Vasu, Eur. J. Med. Chem. 63, 924–934 (2013)
Y.S. Lee, H. Kim, Y.H. Kim, E.J. Roh, H. Han, K.J. Shin, Bioorg. Med. Chem. Lett. 22, 7555–7561 (2012)
Y.K. Abhale, K.K. Deshmukh, A.V. Sasane, A.P. Chavan, P.C. Mhaske, J. Heterocycl. Chem. 53, 229–233 (2016)
P.C. Mhaske, S.H. Shelke, M. Bhoye, V.D. Bobade, J. Heterocycl. Chem. 54, 1590–1597 (2017)
S. Malik, R.S. Bahare, S.A. Khan, Eur. J. Med. Chem. 67, 1–13 (2013)
M.F. Arshad, N. Siddiqui, A. Elkerdasy, H. Abdulmohsen, A.L. Rohaimi, S.A.A. Khan, J. Pharmacol. Toxicol. 9, 132–138 (2014)
J.L. Falco, A. Palomer, A. Guglietta, US 2008/0200473 A12008
F. Hayat, E. Yoo, H. Rhim, H.Y.P. Choo, Bull. Korean Chem. Soc. 34, 495–499 (2013)
R.B. Clark, D. Lamppu, L. Libertine, A. McDonough, A. Kumar, G.L. Rosa, R. Rush, D. Elbaum, J. Med. Chem. 57, 3966–3983 (2014)
J.A. Shiran, A. Yahyazadeh, M. Mamaghani, M. Rassa, J. Mol. Struct. 1039, 113–118 (2013)
M. Brvar, A. Perdith, G. Anderluh, D. Turk, T. Solmajer, J. Med. Chem. 55, 6413–6426 (2012)
C. Araniciu, A.E. Parvu, B. Tiperciuc, M. Palage, S. Oniga, P. Verite, O. Oniga, J Nanomater. Biostruct. 8, 699–709 (2013)
O. Oniga, J.T. Ndongo, C. Moldovan, B. Tiperciuc, S. Oniga, A. Pirnau, L. Vlase, P. Verite, Farmacia 6, 785–797 (2012)
C.B. Mark, J.W. Dale, E.A. Merritt, X. Xin, Chem. Rev. 105, 685–714 (2005)
E. Riego, D. Hernandez, F. Albericio, M. Alvarez, Synthesis 2005, 1907–1922 (2005)
J. Parvate, V.S. Bhagwat, M.M. Doshi, H.L. Mondkar, Indian Drugs 26, 222–226 (1989)
S.H. Shelke, P.C. Mhaske, P. Hande, V.D. Bobade, Phosphorus Sulfur Silicon Relat. Elem. 188, 1262–1270 (2013)
M.R. Shiradkar, K.K. Murahari, G.H. Reddy, S. Tatikonda, A.K. Chakravarthy, P. Dolly, R. Kaur, P. Burange, J. Ghogare, V. Mokalec, M. Rautc, Bioorg. Med. Chem. Lett. 15, 3997–4008 (2007)
Y.K. Abhale, A.V. Sasane, A.P. Chavan, K.K. Deshmukh, S.S. Kotapalli, R. Ummanni, S.F. Sayyad, P.C. Mhaske, Eur. J. Med. Chem. 94, 340–347 (2015)
Y.K. Abhale, A.V. Sasane, A.P. Chavan, S.H. Shekh, K.K. Deshmukh, S. Bhansali, L. Nawale, D. Sarkar, P.C. Mhaske, Eur. J. Med. Chem. 132, 333–340 (2017)
A. Khan, D. Sarkar, J. Microbiol. Methods 73, 62–68 (2008)
R. Singh, L. Nawale, M. Arkile, U. Shedbalkar, S. Wadhwani, D. Sarkar, B. Chopade, Int. J. Antimicrob. Agents 46, 183–188 (2015)
S. Sarkar, D. Sarkar, J. Biomol. Screen. 17, 966–973 (2012)
U. Singh, S. Akhtar, A. Mishra, D. Sarkar, J. Microbiol. Methods 84, 202–207 (2011)
A. Khan, S. Sarkar, D. Sarkar, Int. J. Antimicrob. Agents 32, 40–45 (2008)
T. Mosmann, J. Immunol. Methods 65, 55–63 (1983)
G. Ciapetti, E. Cenni, L. Pratelli, A. Pizzoferrato, Biomaterials 4, 359–364 (1993)
R.C. Hartkoorn, B. Chandler, A. Owen, S.A. Ward, S.B. Squire, D.J. Back, S.H. Khoo, Tuberculosis 87, 248 (2007)
Acknowledgements
The authors are grateful to CSIR-NCL, Pune for supporting the biological activity investigation. Central Analysis Facility, Savitribai Phule Pune University, Pune is also acknowledged for spectral analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shinde, V., Mahulikar, P., Mhaske, P.C. et al. Synthesis and biological evaluation of new 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives. Res Chem Intermed 44, 1247–1260 (2018). https://doi.org/10.1007/s11164-017-3164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3164-4