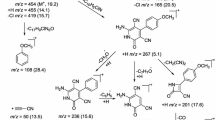
Abstract
The precursor compound 3-fluoro-4-morpholinoaniline (7) is an important intermediate of the antibiotic drug linezolid and was synthesized initially by the substitution of morpholine (5) on 1,2-difluoro-4-nitrobenzene (4) under neat conditions, resulting in 4-(2-fluoro-4-nitrophenyl)morpholine (6) followed by its nitro group reduction with Fe/NH4Cl. A series of new sulfonamides (9a–e) and carbamates (11a–e) have been synthesized in good yields (75–89%) by the reaction of substituted aryl sulfonyl chlorides (8a–e) and substituted aryl/acyclic chloroformates (10a–e) with precursor compound 7, respectively, for biological interest. Structures of the title products were elucidated by spectroscopic data such as IR, NMR (1H and 13C NMR) and mass and elemental analyses. The antimicrobial potency of title products was examined by the screening of growth of zone of inhibition against four bacteria and four fungi, and minimum inhibitory concentration (MIC) was also determined. Most of the compounds showed good to potent antimicrobial activity, whereas the title products, 9d and 9e against bacterial strains, and 9a, 9b, 9d and 11a against fungi, exhibited promising activity in the MIC range of 6.25–25.0 µg/mL. The whole biological activity results revealed that the sulfonamide derivatives behaved as potent antifungal agents as compared to the carbamate derivatives. Molecular docking studies of the crystal structure of topoisomerase II gyrase A complexed with natural inhibitor, clorobiocin (1kzn), using the molecular operating environment (MOE) programme were performed in order to predict the affinity and orientation of the synthesized compounds at the active enzyme site. The test compounds showed good binding affinities and formed hydrogen bonds with a surrounding of amino acids at the active sight, whereas compounds 9d (−189.0 kcal/mol) and 11a (−280.3 kcal/mol) exhibited high binding affinities and good agreement with in vitro antimicrobial screening.




Similar content being viewed by others
References
K. Nagai, T.A. Davies, M.R. Jacobs, P.C. Appelbaum, Antimicrob. Agents Chemother. 46(5), 1273–1280 (2000)
R. Leclerq, P. Courvalin, Agents Chemother. 46(9), 2727–2734 (2002)
W.M. Kirby, Science 99(2579), 452–453 (1944)
K.R. Eriksen, Ugeskr. Laeger 123, 384–386 (1961)
T. Furuya, A.S. Kamlet, T. Ritter, Nature 473, 470–477 (2011)
K. Müller, C. Faeh, F. Diederch, Science 317(5846), 1881–1886 (2007)
A. Abbas, K. Mohsen, H. Ramin, S. Narjes, N.N. Babak, Med. Chem. Res. 21(11), 3532–3540 (2012)
P. Panneerselvam, M. Gnanarupa, N. Priya, K. Ramesh, G. Saravanan, Indian J. Pharm. Sci. 71(4), 428–432 (2009)
I. Alberto, R. Juan, R. Marcela, E. Carlos, Q. Jairo, A. Rodrigo, N. Manuel, C. Justo, V.R. María, A.Z. Susana, I. Braulio, Molecules 18(5), 5482–5497 (2013)
M. Sunny, I.K. Shabana, S.R. Diwan, Chem. Biol. Drug Des. 81(5), 625–630 (2013)
K. Sabine, Z. Alfonso, K. Marcel, B. Reto, S. Leonardo, P. Remo, J. Med. Chem. 50(23), 5833–5839 (2007)
T.M. Nilesh, K.A. Ankar, V. Milan, L. Kartik, J. Chem. Pharm. Res. 8(2), 662–667 (2016)
S. Tsiodras, H.S. Gold, G. Sakoulas, G.M. Eliopoulos, C. Wennersten, L. Venkataraman, R.C. Moellering, M.J. Ferraro, Lancet 358(9277), 207–208 (2001)
J. Seedat, G. Zick, I. Klare, C. Konstabel, N. Weiler, H. Sahly, Antimicrob. Agents Chemother. 50(12), 4217–4219 (2006)
F. Reck, F. Zhou, M. Girardot, G. Kern, C.J. Eyermann, N.J. Hales, R.R. Ramsay, M.B. Gravestock, J. Med. Chem. 48(2), 499–506 (2005)
G. Poce, G. Zappia, G.C. Porretta, B. Botta, M. Biava, Expert Opin. Ther. Pat. 18(2), 97–121 (2008)
O.A. Phillips, E.E. Udo, A.A.M. Ali, S.M. Samuel, Eur. J. Med. Chem. 42(2), 214–225 (2007)
O.A. Phillips, E.E. Udo, M.E. Abdel-Hamid, R. Varghese, Eur. J. Med. Chem. 44(8), 3217–3227 (2009)
S.Y. Kim, H.B. Park, J.H. Cho, K.H. Yoo, C.H. Oh, Bioorg. Med. Chem. 19(9), 2558–2561 (2009)
A.R. Renslo, G.W. Luehr, M.F. Gordeev, Bioorg. Med. Chem. 14(12), 4227–4240 (2006)
T. Komine, A. Kojima, Y. Asahina, T. Saito, H. Takano, T. Shibue, Y. Fukuda, J. Med. Chem. 51(20), 6558–6562 (2008)
Q. Xin, H. Fan, B. Guo, H. He, S. Gao, H. Wang, Y. Huang, Y. Yang, J. Med. Chem. 54(21), 7493–7502 (2011)
A. Kamal, P. Swapna, V.C.R.N.C.S. Rajesh, S.A. Basha, M.P.N. Rao, S. Gupta, Eur. J. med. Chem 62, 661–669 (2013)
K. Xu, P. Wang, X. Xu, F. Chu, J. Lin, Y. Zhang, H. Lei, Res. Chem. Intermed. 41(3), 1385–1411 (2015)
P. Vedavathi, H. Sudhamani, C.N. NRaju, Res. Chem. Intermed. 43(5), 3251–3263 (2017)
H. Sudhamani, S.K.T. Basha, S. Adam, S. Madhusudhana, A.U. Rani, C.N. Raju, Res. Chem. Intermed. 43(1), 103–120 (2017)
K. Berger, B. Petersen, B.H. Pfaue, Arch. Lebensmittelhyg. 37(359), 85–108 (1986)
C.W. Thornber, Chem. Soc. Rev. 8, 563–580 (1979)
R.C. Ogden, C.W. Flexner, Protease inhibitors in AIDS therapy (Marcel Dekker; Inc, New York, 2001), pp. 101–118
I. Nishimori, D. Vullo, A. Innocenti, A. Scozzafava, A. Mastrolorenz, C.T. Supuran, Bioorg. Med. Chem. Lett. 15(17), 3828–3833 (2005)
J.J. Li, D. Anderson, E.G. Burton, J.N. Cogburn, J.T. Collins, D.J. Garland, S.A. Gregory, H.C. Huang, P.C. Isakson, C.M. Koboldt, E.W. Logusch, M.B. Norton, W.E. Perkins, E.J. Reinhard, K. Seibert, A.W. Veenhuizem, Y. Zang, D.B. Reitz, J. Med. Chem. 38(22), 4570–4578 (1995)
D.S. Rao, G. Madhava, C.N. Raju, M. Balaji, S. Madhusudhana, A.U. Rani, Med. Chem. Res. 25(4), 751–768 (2016)
A.E. Boyd, Diabetes 37(7), 847–850 (1988)
K.V. Narayana, D.S. Rao, G. Madhava, N. Venkateswarlu, T. Vijaya, C.N. Raju, Org. Commun. 9(2), 42–53 (2016)
T.S. Claudiu, C. Angela, S. Andrea, Med. Res. Rev. 23(5), 535–538 (2003)
A.K. Ghosh, M. Brindisi, J. Med. Chem. 58, 2895–2940 (2015)
P. Tundo, C.R. McElroy, F. Arico, Synlett 10, 1567–1571 (2010)
L.R. Morgan, R.F. Struck, W.R. Waud, Cancer Chemother. Pharmacol. 64(4), 829–835 (2009)
J. Deng, W. Zhao, W. Yang, React. Funct. Polym. 67, 828–835 (2006)
J.C. Jung, M.A. Avery, Tetrahedron Lett. 47(45), 7969–7972 (2006)
S. Gattinoni, C.D. Simone, S. Dallavalle, Bioorg. Med. Chem. Lett. 20(15), 4406–4411 (2010)
J.A.O. Meara, A. Jakalina, S. La Plante, P.R. Bonneau, R. Coulombe, A.M. Faucher, I. Guse, S. Landry, J. Racine, B. Simoneau, B. Thavonekham, Bioorg. Med. Chem. Lett. 17(12), 3362–3366 (2007)
T. Narasaiah, D.S. Rao, S. Rasheed, G. Madhava, D. Srinivasulu, P.B. Naidu, C.N. Raju, Der Pharmacia Lettre 4(3), 854–862 (2012)
S.V.L. Reddy, S.K.T. Basha, K. Naresh, C.N. Raju, Der Chem. Sin. 4(2), 127–132 (2013)
H. Sudhamani, S.K.T. Basha, S.M.C. Reddy, B. Sreedhar, S. Adam, C.N. Raju, Chem. Intermed. 42(10), 7471–7486 (2016)
R. Menzel, M. Gellert, Adv. Pharmacol. 29A, 201–205 (1994)
A. Maxwell, M. Gellert, Adv. Protein Chem. 38, 69–107 (1986)
L. Brino, A. Urzhumtsev, M. Mousli, C. Bronner, A. Mitschler, P. Oudet, D. Moras, J. Biol. Chem. 275(13), 9468–9475 (2000)
S.K.T. Basha, D.S. Rao, G. Madhava, S.K.T. Basha, M.N. Devamma, S. Madhusudhana, A.U. Rani, C.N. Raju, Phosphorus Sulfur Silicon Relat Elem. 190, 1064–1074 (2015)
B. Song, H. Zhang, H. Wang, S. Yang, L. Jin, D. Hu, L. Pang, W. Xue, J. Agric. Food Chem. 53(20), 7886–7891 (2005)
R. Cruickshank, J.P. Duguid, B.P. Marion, R.H.A. Swain, Medicinal Microbiology, vol. 2, 12th edn. (Churchill Livingstone, London, 1975), pp. 196–202
National Committee for Clinical Standards; 2000. NCCLS. Method for dilution antimicrobial susceptibility test for bacteria that grow aerobically, Approved Standards, 5th edn. (Villanova, PA)
S.Q. Song, L.G. Zhou, D. Li, D. Tang, J.Q. Li, W.B. Jiang, Antifungal activity of five plants from Xinjiang. Nat. Prod. Res. Dev. 16, 157–159 (2004)
National Committee for Clinical Laboratory Standard. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, Approved Standard. Document M27-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1997
National Committee for Clinical Laboratory Standard. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium Forming Filamentous Fungi: Proposed Standard. Document M38-P; National Committee for Clinical Laboratory Standard: Wayne, PA, USA, 1998
National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard, 5th ed.; NCCLS: Villanova, PA, 2000; M7–A5
C. Lino, A. Pietro, B. Marco, D.A. Giuseppe, R. Gabriele, EP Patent 2072505 A2 (2009)
K.V. Balakin, Y.A. Ivanenkov, A.V. Skorenko, Y.V. Nikolsky, N.P. Savchuk, A.A. Ivashchenko, J. Biomol. Screen. 9(1), 22–31 (2004)
D. Lafitte, V. Lamour, P.O. Tsvetkov, A.A. Makarov, M. Klich, P. Deprez, D. Moras, C. Briand, R. Gilli, Biochemistry 41(23), 7217–7223 (2002)
A. Hall, D. Parsonage, L.B. Poole, P.A. Karplus, J. Mol. Biol. 402(1), 194–209 (2010)
J.P. Declercq, C. Evrard, A. Clippe, D.V. Stricht, A. Bernard, B. Knoops, J. Mol. Biol. 311(4), 751–759 (2001)
Acknowledgements
The authors gratefully acknowledge the Department of Bioinformatics and Department of Botany, S. V. University, Tirupati, for their support in the screening of the docking study and biological activity, respectively. The authors also express thanks to Hyderabad Central University and Osmania University for providing instrumentation facilities for recording spectroscopic data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Janakiramudu, D.B., Subba Rao, D., Srikanth, C. et al. Sulfonamides and carbamates of 3-fluoro-4-morpholinoaniline (linezolid intermediate): synthesis, antimicrobial activity and molecular docking study. Res Chem Intermed 44, 469–489 (2018). https://doi.org/10.1007/s11164-017-3114-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3114-1