Abstract
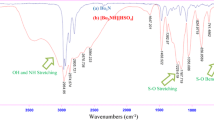
The aim of this work is to introduce a novel and attractive protic acidic ionic liquid as catalyst for organic synthesis. To achieve this aim, N,N-diethyl-N-sulfoethanaminium hydrogen sulfate {[Et3N-SO3H]HSO4} was prepared by reaction of NEt3 with ClSO3H and then with H2SO4. The novel acidic ionic liquid was identified by Fourier-transform infrared (FT-IR), 1H nuclear magnetic resonance (NMR), 13C NMR, and mass spectroscopies. Its catalytic activity was then examined in the cross-aldol condensation reaction of arylaldehydes with cycloalkanones under solvent-free conditions, obtaining α,α′-bis(arylidene)cycloalkanones in high yield after short reaction time.
Graphical Abstract






Similar content being viewed by others
References
B. Kirchner (ed.), Ionic Liquids (Topics in Current Chemistry) (Springer, Berlin, 2010)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis, 2nd edn. (Wiley, Weinheim, 2008)
H. Eshghi, M. Bakavoli, M. Ghasemzadeh, Res. Chem. Intermed. 41, 3999 (2015)
A. Hasaninejad, A. Zare, M. Shekouhy, J. Ameri Rad, J. Comb. Chem. 12, 844 (2010)
A.S. Kucherenko, D.E. Siyutkin, O.V. Maltsev, S.V. Kochetkov, S.G. Zlotin, Russ. Chem. Bull. 61, 313 (2012)
K. Zhuo, Q. Du, G. Bai, C. Wang, Y. Chen, J. Wang, Carbohydr. Polym. 115, 49 (2015)
A. Zare, A.R. Moosavi-Zare, M. Merajoddin, M.A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M.H. Beyzavi, E. Rostami, A. Arghoon, R. Roohandeh, J. Mol. Liq. 167, 69 (2012)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, H.G. Kruger, Z. Asgari, V. Khakyzadeh, M. Kazem-Rostami, J. Org. Chem. 77, 3640 (2012)
S. Salahi, M.T. Maghsoodlou, N. Hazeri, F. Movahedifar, R. Doostmohammadi, M. Lashkari, Res. Chem. Intermed. 41, 6477 (2015)
A. Jamalian, B. Rathman, G.L. Borosky, K.K. Laali, Appl. Catal. A Gen. 486, 1 (2014)
A. Zare, T. Yousofi, A.R. Moosavi-Zare, RSC Adv. 2, 7988 (2012)
H. Naeimi, Z.S. Nazifi, C. R. Chim. 7, 41 (2014)
A.R. Hajipour, M. Karimzadeh, H. Tavallaei, J. Iran. Chem. Soc. 12, 987 (2015)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Appl. Catal. A Gen. 400, 70 (2011)
A.K. Rawat, S. Bhattacharya, S.M.S. Chauhan, Tetrahedron Lett. 55, 4537 (2014)
K. Tanaka, Solvent-Free Organic Synthesis (Wiley, Weinheim, 2009)
E.S. Putilova, G.V. Kryshtal, G.M. Zhdankina, N.A. Troitskii, S.G. Zlotin, Russ. J. Org. Chem. 41, 512 (2005)
A. Khazaei, M. Khazaei, S. Rahmati, J. Mol. Catal. A Chem. 398, 241 (2015)
H. Moghanian, A. Mobinikhaledi, M. Deinavizadeh, Res. Chem. Intermed. 41, 4387 (2015)
A. Zare, M. Merajoddin, A.R. Moosavi-Zare, M. Zarei, M.H. Beyzavi, M.A. Zolfigol, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2154-7
A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, A. Zare, A.R. Pourali, R. Ayazi-Nasrabadi, Synlett 25, 193 (2014)
J. Deli, Pharmazie 39, 539 (1984)
C. Piantadosi, I.H. Hall, J.L. Irvine, G.L. Carlson, J. Med. Chem. 16, 770 (1973)
A.T. Dinkova-Kostova, C. Abeygunawardana, P. Talalay, J. Med. Chem. 41, 5287 (1998)
S.F.P. Braga, É.V.P. Alves, R.S. Ferreira, J.R.B. Fradico, P.S. Lage, M.C. Duarte, T.G. Ribeiro, P.A.S. Júnior, A.J. Romanha, M.L. Tonini, M. Steindel, E.F. Coelho, R.B. de Oliveira, Eur. J. Med. Chem. 71, 282 (2014)
J. Kawamata, K. Inoue, T. Inabe, M. Kiguchi, M. Kato, Y. Taniguchi, Chem. Phys. Lett. 249, 29 (1996)
K. Gangadhara, K. Kaushal, Polymer 36, 1903 (1995)
J.R.A. Dimmock, M.P. Padmanilayam, G. Zello, K.H. Nienaber, T.M. Allen, C.L. Santos, E. De Clercq, J. Balzarini, E.K. Manavathu, J.P. Stables, Eur. J. Med. Chem. 38, 169 (2003)
A. Amoozadeh, E. Tabrizian, S. Rahmani, C. R. Chim. 18, 848 (2015)
E. Tabrizian, A. Amoozadeh, S. Rahmani, M. Salehi, M. Kubicki, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2039-9
A. Zare, M. Merajoddin, A. Hasaninejad, A.R. Moosavi-Zare, V. Khakyzadeh, C. R. Chim. 16, 380 (2013)
A. Solhy, W. Amer, M. Karkouri, R. Tahir, A. El Bouari, A. Fihri, M. Bousmina, M. Zahouily, J. Mol. Catal. A Chem. 336, 8 (2011)
B. Das, P. Thirupathi, I. Mahender, K.R. Reddy, J. Mol. Catal. A Chem. 247, 182 (2006)
A. Lahyani, M. Chtourou, M.H. Frikha, M. Trabelsi, Ultrason. Sonochem. 20, 1296 (2013)
N. Iranpoor, F. Kazemi, Tetrahedron 54, 9475 (1998)
M.A. Bigdeli, G.H. Mahdavinia, S. Jafari, H. Hazarkhani, Catal. Commun. 8, 2229 (2007)
L.-T. An, J.-P. Zou, L.-L. Zhang, Catal. Commun. 9, 349 (2008)
Acknowledgments
The authors thank the Research Council of Payame Noor University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadi, S., Zare, A., Aali-Hosaini, M. et al. Design, characterization, and use of N,N-diethyl-N-sulfoethanaminium hydrogen sulfate {[Et3N-SO3H]HSO4} as a novel and highly efficient catalyst for preparation of α,α′-bis(arylidene)cycloalkanones. Res Chem Intermed 42, 6245–6253 (2016). https://doi.org/10.1007/s11164-016-2458-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2458-2