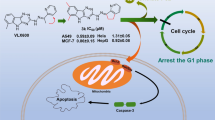
Abstract
A series of novel 1-thiazolyl-5-coumarin-3-yl-pyrazole derivatives (4a–l) were synthesized via one-pot multicomponent reaction of 5-substituted salicylaldehydes (1a–c), 4-hydroxy-6-methyl-2H-pyran-2-one (2) and 2-hydrazinyl-4-arylthiazoles (3a–d) in acetonitrile using a catalytic amount of piperidine under reflux conditions. This multicomponent approach has advantages such as reduced reaction time and a high product yield percentage when compared with corresponding multistep approaches. All the synthesized compounds were evaluated for their cytotoxic activity against Hep G2 (hepatocellular liver carcinoma) and MCF-7 (breast cancer) cell lines and compared with the standard drug Doxorubicin. Among all the compounds, compounds 4d against Hep G2, 4k against MCF-7 and 4e against both Hep G2 & MCF-7 showed excellent cytotoxic activity.


Similar content being viewed by others
References
S. Eckhardt, Anti-cancer agents. Curr. Med. Chem. 2, 419 (2002)
K.H. Altmann, Curr. Opin. Chem. Biol. 5, 424 (2001)
S.L. Zhu, Y. Wu, C.J. Liu, C.Y. Wei, J.C. Tao, H.M. Liu, Eur. J. Med. Chem. 65, 70 (2013)
J.M. Lee, Y. Na, H. Han, S. Chang, Chem. Soc. Rev. 33, 302 (2004)
S.J. Broadwater, S.L. Roth, K.E. Price, M. Kobaslija, D. Tyler, McQuade. Org. Biomol. Chem. 3, 2899 (2005)
I. Koca, A. Ozgur, K.A. Coskun, Y. Tutar, Bioorg. Med. Chem. 21, 3859 (2013)
J. Rangaswamy, H. VijayKumar, S.T. Harini, N. Naik, J. Arab. Chem. (2013). doi:10.1016/j.arabjc.2013.10.012
O.I. el-Sabbagh, M.M. Baraka, S.M. Ibrahim, C. Pannecouque, G. Andrei, R. Snoeck, J. Balzarini, A.A. Rashad, Eur. J. Med. Chem. 44, 3746 (2009)
R.B. Pathak, P.T. Chovatia, H.H. Parekh, Bioorg. Med. Chem. Lett. 22, 5129 (2012)
S. Vishwanath, V.K. Honnaiah, N. Nagaraja, J. Pharm. Res. 6, 785 (2013)
S.Y. Jadhav, S.P. Shirame, S.D. Kulkarni, S.B. Patil, S.K. Pasale, R.B. Bhosale, Bioorg. Med. Chem. Lett. 23, 2575 (2013)
M. Abdel-Aziz, Gel-D Abuo-Rahma, A.A. Hassan, Eur. J. Med. Chem. 44, 3480 (2009)
K.M. Dawood, T.M. Eldebss, H.S. El-Zahabi, M.H. Yousef, P. Metz, Eur. J. Med. Chem. 70, 740 (2013)
N.C. Desai, V.V. Joshi, K.M. Rajpara, H.V. Vaghani, H.M. Satodiya, J. Fluor. Chem. 142, 67 (2012)
R. Aggarwal, S. Kumar, P. Kaushik, D. Kaushik, G.K. Gupta, Eur. J. Med. Chem. 62, 508 (2013)
B.F. Abdel-Wahab, H.A. Abdel-Aziz, E.M. Ahmed, Eur. J. Med. Chem. 44, 2632 (2009)
P. Manojkumar, T.K. Ravi, S. Gopalakrishnan, Eur. J. Med. Chem. 44, 4690 (2009)
X.Q. Wu, C. Huang, Y.M. Jia, B.A. Song, J. Li, X.H. Liu, Eur. J. Med. Chem. 74, 717 (2014)
S. Bondock, T. Naser, Y.A. Ammar, Eur. J. Med. Chem. 62, 270 (2013)
B.L. Hemali, R.G. Rakesh, D.I. Brahmbhatt, Chin. Chem. Lett. 24, 227 (2013)
R. Romagnoli, P.G. Baraldi, M.K. Salvador, M.E. Camacho, D. Preti, M.A. Tabrizi, M. Bassetto, A. Brancale, E. Hamel, R. Bortolozzi, G. Basso, G. Viola, Bioorg. Med. Chem. 20, 7083 (2012)
K.V. Sashidhara, A. Kumar, M. Kumar, J. Sarkar, S. Sinha, Bioorg. Med. Chem. Lett. 20, 7205 (2010)
H.I. El-Subbagh, A.M. Al-Obaid, Eur. J. Med. Chem. 31, 1017 (1996)
C.F. Xiao, L.Y. Tao, H.Y. Sun, W. Wei, Y. Chen, L.W. Fu, Y. Zou, Chin. Chem. Lett. 21, 1295 (2010)
M. Andreani, A. Rambaldi, A. Leoni, G.Pifferi Locatelli, J. Pharm. Belg. 49, 308 (1994)
R.K. Goel, R.N. Maiti, M. Manickam, A.B. Ray, Indian J. Exp. Biol. 35, 1080 (1997)
J.S. Barradas, M.I. Errea, N.B. D’Accorso, C.S. Sepúlveda, E.B. Damonte, Eur. J. Med. Chem. 46, 259 (2011)
J.C. Trivedi, J.B. Bariwal, K.D. Upadhyay, Y.T. Naliapara, S.K. Joshi, C.C. Pannecouque, E.D. Clercqd, A.K. Shaha, Tetrahedron Lett. 48, 8472 (2007)
V. Jaishree, N. Ramdas, J. Sachin, B. Ramesh, J. Saudi Chem. Soc. 16, 371 (2012)
C.-R. Wua, M.-Y. Huang, Y.-T. Lin, H.-Y. Ju, H. Ching, Food Chem. 104, 1464 (2007)
N. Siddiqui, W. Ahsan, Eur. J. Med. Chem. 45, 1536 (2010)
K.M. Amin, D.E. Rahman, Y.A. Al-Eryani, Bioorg. Med. Chem. 16, 537 (2008)
M. Andreani, A. Rambaldi, A. Locatelli, S. Cristoni, G. Malandrino, Pifferi. Acta Pharm. Nord. 4, 93 (1992)
M.J. Lee, F.P. Chou, T.H. Tseng, M.H. Hsieh, M.C. Lin, C.J. Wang, J. Agric. Food Chem. 50, 2130 (2002)
A. Alizade, R. Ghanbaripour, Helv. Chim. Acta 96, 473 (2013)
A. Alizade, R. Ghanbaripour, Synth. Commun. 44, 1635 (2014)
G. Rajitha, B. Janardhan, V. Ravibabu, P. Mahendar, H. Sairengpuii, B. Rajitha, A. Sadanandam, B. Siddhardha, Bioorg. Med. Chem. Lett. 24, 4239 (2014)
B. Janardhan, G. Rajitha, V. Ravibabu, B. Rajitha, J. Chem. Sci. 125, 843 (2013)
K.B. Subrahmanya, B.H. Shivarama, Phosphorus. Sulfur Silicon Relat Elem 179, 1019 (2004)
F. Denizot, R. Lang, J. Immunol. Methods 89, 271 (1986)
S. Myadaraboina, M. Alla, V. Saddanapu, V.R. Bommena, A. Addlagatta, Eur. J. Med. Chem. 45, 5208 (2010)
R.S. Upadhyaya, J.K. Vandavasi, V.N. Rao, S. Vivek, S.S. Dixit, J. Chattopadhyaya, J. Bioorg. Med. Chem. 17, 2830 (2009)
Acknowledgments
We would like to thank Prof. A. Sadanandam, Biotechnology Department, Kakatiya University for performing cytotoxic activity. We would like to thank the Director, National Institute of Technology, Warangal for providing research facilities. The author’s RV, RD and RG thank CSIR-UGC New Delhi, India for research fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is a link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Velpula, R., Deshineni, R., Gali, R. et al. One-pot multicomponent synthesis of novel 1-thiazolyl-5-coumarin-3-yl-pyrazole derivatives and evaluation of their cytotoxic activity. Res Chem Intermed 42, 1729–1740 (2016). https://doi.org/10.1007/s11164-015-2114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2114-2