Abstract
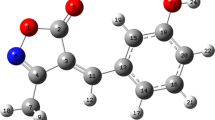
A three-component, NBS-promoted synthesis of α,β-unsaturated isoxazol-5(4H)-ones by reaction of aromatic aryl or hetero-aryl aldehydes, hydroxylamine hydrochloride, and 1,3-dicarbonyl compounds (ethyl acetoacetate or ethyl 4-chloroacetoacetate), under mild reaction conditions at room temperature is described. This simple, efficient, and clean reaction is an expeditious means of obtaining the corresponding isoxazol-5(4H)-one derivatives in good to high yields. Geometrical properties and vibrational wavenumbers of 3-methyl-4-(thiophen-2-ylmethylene)isoxazol-5(4H)-one (MTISO) were predicted by use of density functional theory (DFT) by use of the B3LYP level with the 6-311++G(d,p) and 6-311++G(2d,p) basis sets. Results indicate that the B3LYP method enables satisfactory prediction of vibrational frequencies and structural data. The absorption spectra of MTISO in solvents of different polarity were studied at room temperature. The UV–visible spectrum of the compound was recorded and such electronic properties as the energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) were determined by the time-dependent DFT (TD-DFT) approach. The stability of the molecule arising from hyper-conjugative interaction and charge delocalization was studied by NBO analysis. A molecular electrostatic potential map (MEP) of the compound was also studied to predict reactive sites. Reactivity descriptors, Fukui functions, and electrophilic sites were found and are discussed. The thermal stability of MTISO was studied by thermogravimetric analysis (TGA).
















Similar content being viewed by others
References
J. Han, H. Guo, X.G. Wang, M.L. Pang, J.B. Meng, Chin. J. Chem. 25, 129 (2007)
M. Pineiro, T.M.V.D. Pinho-e-Melo, Eur. J. Org. Chem. 31, 5287 (2009)
S. Biju, M.L. Reddy, R.O. Freire, Inorg. Chem. Commun. 10, 393 (2007)
E. Aret, H. Meekes, E. Vlieg, G. Deroover, Dyes Pigment. 72, 339 (2007)
X.H. Zhang, Y.H. Zhan, D. Chen, F. Wang, L.Y. Wang, Dyes Pigment. 93, 1408 (2012)
B. Kafle, N.G. Aher, D. Khadka, H. Park, H. Cho, Chem. Asian J. 6, 2073 (2011)
H. Kano, I. Adachi, R. Kido, K. Hirose, J. Med. Chem. 10, 411 (1967)
T. Karabasanagouda, A.V. Adhikari, M. Girisha, Indian J. Chem. 48B, 430 (2009)
A. Mor, S. Ahn, P. Izmirly, S. Reddy, J. Greenberg, C.O. Bingham, P.B. Rosenthal, Indian Phytopathol. 59, 370 (2006)
J. Getal, J. Antibiot. Antibiot. 28, 91 (1975)
K. Bowden, G. Crank, W.J. Ross, J. Chem. Soc. C. 172 (1968). doi:10.1039/J39680000172
C.H. Stammer, A.N. Wilson, C.F. Spencer, F.W. Bachelor, F.W. Holly, K. Folkers, J. Am. Chem. Soc. 79, 3236 (1957)
Q. Liu, Y.N. Zhang, Bull. Korean Chem. Soc. 32, 3559 (2011)
Q. Liu, X. Hou, Phosphorus, Sulfur Silicon. Relat. Elem. 187, 448 (2012)
Q. Liu, R.T. Wu, J. Chem. Res. 35, 598 (2011)
M. Mirzadeh, G.H. Mahdavinia, Eur. J. Chem. 9, 425 (2012)
S. Fozooni, N. Gholam Hosseinzadeh, H. Hamidian, M.R. Akhgar, J. Braz. Chem. Soc. 24, 1649 (2013)
F. Saikh, J. Das, S. Ghosh, Terahedron Lett. 54, 4679 (2013)
K. Ablajan, H. Xiamuxi, Synth. Commun. 42, 1128 (2012)
Q.F. Cheng, X.Y. Liu, Q.F. Wang, L.S. Liu, W.J. Liu, Q. Lin, X.J. Yang, Chin. J. Org. Chem. 29, 1267 (2009)
K. Ablajan, H. Xiamuxi, Chin. Chem. Lett. 22, 151 (2011)
Y.Q. Zhang, J.J. Ma, C. Wang, J.C. Li, D.N. Zhang, X.H. Zang, J. Li, Chin. J. Org. Chem. 28, 141 (2008)
Y.Q. Zhang, C. Wang, M.Y. Zhang, P.L. Cui, Y.M. Li, X. Zhou, J.C. Li, Chin. J. Org. Chem. 28, 914 (2008)
H. Kiyani, Org. Chem. Indian J. 13, 97 (2013)
H. Kiyani, F. Ghorbani, Heterocycl. Lett. 3, 145 (2013)
H. Kiyani, F. Ghorbani, Heterocycl. Lett. 3, 359 (2013)
H. Kiyani, F. Ghorbani, Open. J. Org. Chem. 1, 5 (2013)
H. Kiyani, F. Ghorbani, Elixir Org. Chem. 58A, 14948 (2013)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1411-x
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. (2013). doi:10.1016/j.jscs.2013.11.002
M.J. Frisch et al., Gaussian 03, Revision C.01 (Gaussian, Inc., Wallingford, 2004), p. 255
A.D. Becke, J. Chem. Phys. 98, 5648 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
B. Miehlich, A. Savin, H. Stoll, H. Preuss, Chem. Phys. Lett. 157, 200 (1989)
R. Dennington, T. Keith, Millam Gaussview Version 5. Semichem Inc., Shawnee Mission KS (2009)
A.P. Scott, L. Radom, J. Phys. Chem. 100, 16502 (1996)
M. Karabacak, M. Cinar, M. Kurt, Spectrochim. Acta 74A, 1197 (2009)
E.D. Glendening, A.E. Reed, J.E. Carpenter, F. Weinhold, NBO Version 3.1 (Gaussian Inc., Pittsburgh, 2003)
C. Gündoğdu, S. Alp, Y. Ergün, B. Tercan, T. Hökelek, Acta Cryst. E67, 1321 (2011)
Q. Cheng, X.Y. Xu, L.S. Liu, L. Zhang, Acta Cryst. E65, 3012 (2009)
J. Mohan, Organic Spectroscopy—Principle and Applications, 2nd edn. (Narosa Publishing House, New Delhi, 2000)
G. Brancatelli, G. Bruno, F. Nicolò, M. Cordaro, G. Grassi, F. Risitano, A. Scala, J. Mol. Struct. 998, 157 (2011)
R.M. Silverstein, G.C. Basseler, C. Morill (eds.), Spectroscopic Identification of Organic compounds (Wiley, New York, 1981)
T. Kupka, R. Wrzalik, G. Pasterna, K. Pasterny, J. Mol. Struct. 616, 17 (2002)
D.K. Singh, S.K. Srivastava, A.K. Ojha, B.P. Asthana, J. Mol. Struct. 892, 384 (2008)
A. Coruh, F. Yilmaz, B. Sengez, M. Kurt, M. Cinar, M. Karabacak, Struct. Chem. 22, 45 (2011)
M. Szafran, A. Komasa, E.B. Adamska, J. Mol. Struct. (Theochem) 827, 101 (2007)
N. Günay, H. Pir, D. Avcı, Y. Atalay, J. Chem. 2013, 1 (2012)
J. Choo, S. Kim, H. Joo, Y. Kwon, J. Mol. Struct. (Theochem) 587, 1 (2002)
R.S. Mulliken, J. Chem. Phys. 23, 1833 (1995)
R. Meenakshi, L. Jaganathana, S. Gunasekaranb, S. Srinivasan, Mol. Simul. 36, 425 (2010)
H.O. Kalinowski, S. Berger, S. Braun, Carbon-13 NMR Spectroscopy (Wiley, Chichester, 1988)
N. Subramania, N. Sundaraganesan, J. Jayabharathi, Spectrochim. Acta A 76, 259 (2010)
E. Scrocco, J. Tomasi, Adv. Quant. Chem. 11, 115 (1978)
N.M. O’Boyle, A.L. Tenderholt, K.M. Langner, J. Comp. Chem. 29, 839 (2008)
K. Fukui, Science 218, 747 (1982)
R.E. Stratmann, G.E. Scuseria, M.J. Frisch, J. Chem. Phys. 109, 8218 (1998)
B. Mennucci, J. Tomasi, J. Chem. Phys. 106, 5151 (1997)
A. Airinei, M. Homocianu, D. Dorohoi, J. Mol. Liq. 157, 13 (2010)
D.A. Kleinman, Phys. Rev. 126, 1977 (1962)
A. Chandran, S. Mary, H.T. Varghese, C.Y. Panicker, T.K. Manojkumar, C.V. Alsenoy, G. Rajendran, ISRN Anal. Chemistry. 2012, 1 (2011)
C. Adant, M. Dupuis, L. Bredas, Int. J. Quantum Chem. 56, 497 (2004)
M.A. Palafox, Int. J. Quantum Chem. 77, 661 (2000)
J. Padmanabhan, R. Parthasarathi, V. Subramanian, P.K. Chattaraj, J. Phys. Chem. A 111, 1358 (2007)
A.P. Garrido, A.M. Helguera, A.A. Guillén, M.N.D.S. Cordeirom, A.G. Escudero, Bioorg. Med. Chem. 17, 896 (2009)
R. Parthasarathi, J. Padmanabhan, V. Subramanian, U. Sarkar, B. Maiti, P.K. Chattraj, Internet Electron. J. Mol. Des. 2, 798 (2003)
R.G. Parr, W. Yang, J. Am. Chem. Soc. 106, 4049 (1984)
Acknowledgments
Financial support by Damghan University is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kiyani, H., Kanaani, A., Ajloo, D. et al. N-bromosuccinimide (NBS)-promoted, three-component synthesis of α,β-unsaturated isoxazol-5(4H)-ones, and spectroscopic investigation and computational study of 3-methyl-4-(thiophen-2-ylmethylene)isoxazol-5(4H)-one. Res Chem Intermed 41, 7739–7773 (2015). https://doi.org/10.1007/s11164-014-1857-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1857-5