Abstract
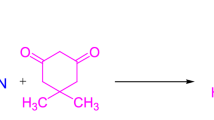
Lactose has been used as a mild, efficient, green, and inexpensive catalyst for the synthesis of tetrahydrobenzo[b]pyran derivatives via a one-pot, three-component condensation between aryl aldehydes, malononitrile, and dimedone in H2O:EtOH at 60 °C. This method offers a considerable number of advantages including short reaction time, high to quantitative yields, low cost, easy accesses, and simple work-up.




Similar content being viewed by others
References
G.P. Ellis, in The Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones, ed. by A. Weissberger, E.C. Taylor (Wiley, New York, 1977), p. 11
A. Shaabani, A. Maleki, A.H. Rezayan, A. Sarvary, Mol. Divers. 15, 41 (2011)
A. Shaabani, M. Mohammadpour Amini, S. Ghasemi, R. Ghadari, A.H. Rezayan, Y. Fazaeli, S. Feizi, Chem. Pharm. Bull. 58, 270 (2010)
S. Banerjee, A. Horn, H. Khatri, G.A. Sereda, Tetrahedron Lett. 52, 1878 (2011)
S. Banerjee, G. Sereda, Tetrahedron Lett. 50, 6959 (2009)
L. Alvey, S. Prado, V. Huteau, B. Saint-JonisMichel, S. Koch, S.M. Cole, F.T. Tillequin, Y.L. Janin, Bioorg. Med. Chem. 16, 8264 (2008)
T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Bioorg. Med. Lett. 19, 1139 (2009)
T. Narender, S. Shweta Gupta, Bioorg. Med. Chem. Lett. 14, 3913 (2004)
L. Lakshmi, K. Pandey, A. Kapil, N. Singh, M. Samant, A. Dube, Phytomedicine 14, 36 (2007)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
A.M. El-Agrody, M.H. El-Hakium, M.S. Abd El-Latif, A.H. Fekry, E.S.M. El-Sayed, K.A. El-Gareab, Acta Pharm. 50, 111 (2000)
C.S. Konkoy, D.B. Fick, S.X. Cai, N.C. Lan, J. Keana, Chem. Abstr. 134, 29313a (2001)
D. Arnesto, W.M. Horspool, N. Martin, A. Ramos, C. Seaone, J. Org. Chem. 54, 3069 (1989)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625 (2004)
S.J. Tu, H. Jiang, Q.Y. Zhung, C.B. Miu, D.Q. Shi, X.S. Wang, Y. Gao, Chin. J. Org. Chem. 23, 488 (2003)
T.S. Jin, A.Q. Wang, F. Shi, L.S. Han, L.B. Liu, T.S. Li, ARKIVOC xiv, 78 (2006)
S. Balalaie, M. Shiekh-Ahmadi, M. Barazjanian, Cat. Commun. 8, 1724 (2007)
S. Abdolmohammadi, S. Balalaie, Tetrhedron Lett. 48, 3299 (2007)
S. Gao, C.H. Tsai, C. Tseng, C.F. Yao, Tetrahedron 64, 9143 (2008)
M. Seifi, H. Sheibani, Catal. Lett. 126, 275 (2008)
R. Hekmatshor, S. Majedi, K. Bakhtiari, Cat. Commun. 9, 307 (2008)
Y.M. Ren, C. Cai, Cat. Commun. 9, 1017 (2008)
M.M. Heravi, B.A. Jani, F. Derikvand, F.F. Bamoharram, H.A. Oskooie, Cat. Commun. 10, 272 (2008)
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50, 4125 (2009)
G. Shabitha, K. Harundhathi, K. Sudhakar, B.S. Sartry, J.S. Yadav, Synth. Commun. 39, 433 (2009)
W.O. Sun, P. Zhang, J. Fan, S.H. Chen, Z.H. Zhang, Synth. Commun. 40, 587 (2010)
L.M. Wang, J.H. Shao, H. Tian, Y.H. Wang, B. Liu, J. Fluor. Chem. 127, 97 (2006)
M.M. Khodaei, K. Bahrami, A. Farrokhi, Synth. Commun. 40, 1492 (2010)
D. Fang, H.B. Zhang, Z.L. Liu, J. Heterocycl. Chem. 47, 63 (2010)
Y. Li, H. Chen, C. Shi, S. Ji, J. Comb. Chem. 12, 231 (2010)
S.S. Katkar, M.K. Lande, B.R. Arbad, S.T. Gaikwad, Chin. J. Chem. 29, 199 (2011)
J. Zheng, Y.Q. Li, Sch. Res. Libr. 3, 381 (2011)
P.P. Salvi, A.M. Mandhare, A.S. Sartape, D.K. Pawar, S.H. Han, S.S. Kolekar, C. R. Chim. 14, 878 (2011)
P.A. Grieco (ed.), Organic Synthesis in Water (Blackie Academic and Professional, London, 1998)
K. Ramesh, K. Karnakar, G. Satish, Y.V.D. Nageswar, Chin. Chem. Lett. 23, 1331 (2012)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Mollashahi, Chin. J. Catal. (2014). doi:10.1016/S1872-2067(14)60003-6
M. Kangani, N. Hazeri, M. Maghsoodlou, S. Salahi, Res. Chem. Intermed. (2013). doi:10.107/s11164-013-1365-z
M.R. Mousavi, N. Hazeri, M.T. Maghsoodlou, S. Salahi, S.M. Habibi-Khorassani, Chin. Chem. Lett. 24, 411 (2013)
N. Hazeri, S.S. Sajadikhah, M.T. Maghsoodlou, M. Norouzi, M. Moin, S. Mohamadian-Souri, J. Chem. Res. 3, 550 (2013)
T.S. Jin, A.Q. Wang, X. Wang, J.S. Zhang, T.S. Li, Synlett 5, 871 (2004)
S. Gurumurthi, V. Sundari, R. Valliappan, Eur. J. Chem. 6, 466 (2009)
N.M.A. El -Rahaman, A.A. El-Kateb, M.F. Mady, Synth. Commun. 37, 3961 (2007)
Acknowledgments
We are thankful to the University of Sistan and Baluchestan Research Council for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeh, F.N., Maghsoodlou, M.T., Hazeri, N. et al. A facile and efficient synthesis of tetrahydrobenzo[b]pyrans using lactose as a green catalyst. Res Chem Intermed 41, 5907–5914 (2015). https://doi.org/10.1007/s11164-014-1710-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1710-x