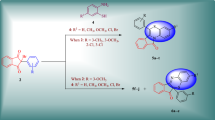
Abstract
New functionalized 1,2,3-triazoloquinolines were achieved by intramolecular azide–alkyne Huisgen [3+2] cycloaddition. These derivatives were synthesized via the key Baylis–Hillman adduct under mild, neutral conditions in short duration and consistently good yield. The structures of final compounds were characterized by spectral analysis. Antifungal activities of these analogues against Trichophyton mentagrophytes, Candida albicans, and Aspergillus niger were also assayed.

Similar content being viewed by others
References
M. Jilino, M.F.G. Stevens, J. Chem. Soc. Perkin Trans. 1, 1677–1684 (1998)
M.D. Chen, S.J. Lu, G.P. Yuag, S.Y. Yang, X.L. Du, Hetrocycl. Commun. 6, 421–426 (2000)
R.J. Singh, Rasayan J. Chem. 2, 706–708 (2009)
M.I. Husain, M. Amir, J. Indian Chem. Soc. 63, 317–319 (1986)
A. Passannanti, P. Diana, P. Barraja, F. Mingoia, A. Lauria, G. Cirrincione, Heterocycles 48, 1229–1235 (1998)
C. Temle Jr, Chem. Hetyrocycl. Compd. 37, 1 (1981)
J.B. Polya, M. Woodruff, Aust. J. Chem. 26, 1585 (1973)
C. Calzolari, L. Favretto, Analyst 93, 494 (1968)
M. Mokotoff, M. Jhao, S.M. Roth, J.A. Shelley, J.N. Slavoskiand, N.M. Kouttab, J. Med. Chem. 33, 354 (1990)
S. Manfredini, C.B. Vicentini, M. Manfrini, N. Bianchi, C. Rutigliano, C. Mischiati, R. Gambari, Bioorg. Med. Chem. 8, 2343–2346 (2000)
S. Danoun, G. Baziard-Mouysset, J. Stigliani, M. Payard, M. Selkti, B. Viossat, A. Thomas, Hetrocycl. Commun. 4, 45–51 (1998)
S. Pautus, S. Yee, M. Jayne, M.P. Coogan, C. Simons, Bioorg. Med. Chem. 14, 3653–4643 (2006)
D. Kim, J. Kim, H. Park, Bioorg. Med. Chem. 12, 2014–2020 (2004)
P. Zoumpoulakis, C. Camoutsis, G. Pairas, M. Sokovic, J. Glamoclija, C. Potamitis, A. Pitsas, Bioorg. Med. Chem. 20, 1569–1583 (2012)
T. Weide, S.A. Saldanha, D. Minod, T.P. Spicer, J.R. Fotsing, M. Spaargaren, J.-M. Frere, C. Bebrone, K.B. Sharpless, P.S. Hodder, V.V. Fokin, ACS Med. Chem. Lett. 1(4), 150–154 (2010)
G. Biagi, V. Calderone, I. Giorgi, O. Livi, E. Martinotti, A. Martelli, A. Nrdi, Farmaco 59, 397–404 (2004)
N. Saravanan, M. Arthanareeswari, P. Kamaraj, Int. J. Chem. 34, 1143–1147 (2013) ISSN: 2051-3240
L. Ackermann, H.K. Potukuchi, Org. Biomol. Chem. 8, 4503–4513 (2010)
C.W. Tornoe, C. Christensen, M. Meldal, Chem. Rev. 108, 2952–3015 (2008)
P. Thirumuruga, D. Matosiuk, K. Jozwaik, Chem. Rev. 113, 4905-4979 (2013)
C.H. Wong, S.C. Zimmerman, Chem. Commun. 49, 1679–1695 (2013)
L. Casarrubios, M.C. de la Torre, M.A. Sierra, Chem. Eur. J. 19, 3534–3541 (2013)
H.Y. Hsieh, W.C. Lee, G.C. Senadi, W.P. Hu, J.J. Liang, T.R. Tsai, Y.W. Chou, K.K. Kuo, C.Y. Chen, J.J. Wang, J. Med. Chem. 56, 5422–5435 (2013)
M.E. Meza Avina, M.K. Patel, C.B. Lee, T.J. Dietz, M.P. Croatt, Org. Lett. 13, 2984–2987 (2011)
R.V. Patel, S.W. Park, Eur. J. Med. Chem. 71, 24–30 (2014)
D. Basavaiah, P. Darma Rao, R.S. Hyma, Tetrahedron 52, 8001–8062 (1996)
S.E. Drewes, G.H.P. Roos, Tetrahedron 44, 4653–4670 (1988)
G.P. Black, F. Dinon, S. Fratucello, P.J. Murphy, M. Nielsen, H.L. Williams, Tetrahedron Lett. 38, 8561–8564 (1997)
L.J. Brzezinski, S. Rafel, J.W. Leahy, Tetrahedron 53, 16423–16434 (1997)
O. Meth-Cohn, B. Narine, B. Tarnowski, J. Chem. Soc. Perkin Trans. 1, 1520–1530 (1981)
M. Zahid, V.O. Iaroshenko, A. Saghyan, C. Fischer, Tetrahedron 69, 3451–3458 (2013)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2596–2599 (2002)
B.A.A. Skaggs et al., J. Clin. Microbiol. 38, 2254–2260 (2000)
Acknowledgments
The authors are grateful to the Department of Chemistry, SRM University for providing the laboratory facilities to carry out the research work. The authors are also grateful to Dr. Suresh for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saravanan, N., Arthanareeswari, M., Kamaraj, P. et al. Efficient synthesis via azide–alkyne Huisgen [3+2] cycloaddition reaction and antifungal activity studies of novel triazoloquinolines. Res Chem Intermed 41, 5379–5388 (2015). https://doi.org/10.1007/s11164-014-1638-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1638-1