Abstract
Transesterification of β-ketoesters with different alcohols has been studied under conventional and unconventional conditions using the simple desktop chemical cesium carbonate as catalyst. These methods enabled transesterification of β-ketoesters in good yields with dramatic rate acceleration and reduced reaction times. The procedures which used unconventional methods, for example sonication and microwave irradiation, are highly promising compared with conventional procedures.
Graphical Abstract

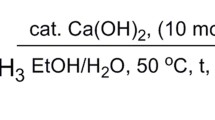
Scheme 1 General Equation for a transesterification reaction

Scheme 2 Transesterification of β-ketoesters







Similar content being viewed by others
References
R.C. West (ed.), CRC Handbook of Chemistry and Physics, 62nd edn. (CRC Press, Boca Raton, 1981). p. B-91. ISBN 0-8493-0462-8
D.R. Lide (ed.), Handbook of Chemistry and Physics, 83rd edn. (CRC Press LLC, Boca Raton, 2002–2003)
C. Galli, Org. Prep. Proced. Int. 24, 287 (1992)
J.A. Cella, S.W. Bacon, J. Org. Chem. 49, 1122 (1984)
J.P. Wolfe, S.L. Buchwald, J. Org. Chem. 65, 1144 (2000)
G. Dijkstra, W.H. Kruizinga, R.M. Kellogg, J. Org. Chem. 52, 4230 (1987)
M. Escudero, L.D. Kremenchuzky, I.A. Perillo, H. Cerecetto, M. Blanco, Synthesis 4, 571 (2010)
B.U.W. Maes, K.T.J. Loones, T.H.M. Jonckers, G.L.F. Lemière, R.A. Dommisse, A. Haemers, Synlett 12, 1995 (2002)
M. Catellani, C. Catucci, G. Celentano, R. Ferraccioli, Synlett 6, 803 (2001)
R.N. Salvatore, A.S. Nagle, K.W. Jung, J. Org. Chem. 67, 674 (2002)
S.W. Wright, D.L. Hageman, L.D. McClure, J. Org. Chem. 59, 6095 (1994)
E. Maerten, F. Hassouna, S. Couve-Bonnaire, A. Mortreux, J.F. Carpentier, Y. Castanet, Synlett 12, 1874 (2003)
J.P. Parrish, B. Sudaresan, K.W. Jung, Synth. Commun. 29, 4423 (1999)
R. Gujadhur, D. Venkataraman, J.T. Kintigh, Tetrahedron Lett. 42, 4791 (2001)
T. Sato, J. Otera, Synlett 4, 336 (1995)
M.T. Honaker, J. Sandefur, J.L. Hargett, A.L. McDaniel, R.N. Salvatore, Tetrahedron Lett. 44, 8373 (2003)
Y.J. Wu, H. He, Synlett 12, 1789 (2003)
A. Kondoh, H. Takami, H. Yorimitsu, K. Oshima, J. Org. Chem. 70, 6468 (2005)
B. Karimi, F.K. Estanhani, R. Soc. Chem. 55, 5556 (2009)
L. Lie, G. Rao, H.L. Sun, J.L. Zhang, Adv. Synth. Catal. 352, 2371 (2010)
J.S. Witzemann, W.D. Nottingham, J. Org. Chem. 54, 2815 (1989)
J. Otera, Chem. Rev. 93, 1449 (1993)
L. Hintermann, A. Togni, Helv. Chim. Acta 83, 2425 (2000)
X.-C. He, B. Wang, G. Yu, D. Bai, Tetrahedron Asymmetry 12, 3213 (2001)
T. Sugimura, S. Nakagawa, A. Tai, Bull. Chem. Soc. Jpn. 75, 355 (2002)
D. Franco, A. Riahi, F. Henin, J. Muzart, E. Dunach, Eur. J. Org. Chem. 14, 2257 (2002)
N. Nishiwaki, D. Nishida, T. Ohnishi, F. Hidaka, S. Shimizu, M. Tamura, K. Hori, Y. Tohda, M. Ariga, J. Org. Chem. 68, 8650 (2003)
K. Majima, R. Takita, A. Okada, T. Ohshima, M. Shibasaki, J. Am. Chem. Soc. 125, 15837 (2003)
C.A.M. Fraga, L.H.P. Teixeira, C.M.S. Menezes, C.M.R. Sant Anna, M.C.K.V. Ramos, F.R. Aquino Neto, E. Barreiro, Tetrahedron 60, 2745 (2004)
M. Gianotti, G. Martelli, G. Spunta, E. Campana, M. Panunzio, M. Mendozza, Synth. Commun. 30, 1725 (2000)
J. Otera, Chem. Rev. 93, 1449 (1993)
S. Benetti, R. Romagnoli, C. De Risi, S. Giampiera, Z. Vinicio, Chem. Rev. 95, 1065 (1995)
G. Krishnaiah, B. Sandeep, D. Kondhare, K.C. Rajanna, J.N. Reddy, Y.R. Rao, P.K. Zhubaidha, Tetrahedron Lett. 54, 703 (2013)
M. Bender, J. Figuera, J. Am. Chem. Soc. 75, 6304 (1953)
D. Bradley, R.C. Mehrotra, I. Rothwell, A. Singh, Alkoxo and Aryloxo Derivatives of Metals (Academic Press, San Diego, 2001)
T.G. Leighton, The Acoustic Bubble (Academic, London, 1994)
E.B. Flint, K.S. Suslick, Science 253, 1397 (1991)
W.B. McNamara, Y. Didenko, K.S. Suslick, in Sonochemistry and Sonoluminescence, Proceedings of the NATO Advanced Study Institute, Series C, vol 524 (Kluwer, Dordrecht, 1999)
K.S. Suslick (ed.), Ultrasound: Its Chemical, Physical, and Biological Effects (VCH, New York, 1988)
S.M. Haile, D.W. Johnson, G.H. Wiseman, H.K. Bowen, J. Am. Ceram. Soc. 72, 2004 (1989)
Acknowledgments
The authors are indebted to CSIR, New Delhi (India) and UGC (New Delhi) for financial support in the form of an SRF Fellowship to G. Krishnaiah and a JRF Fellowship to P. Srinivas. The authors gratefully acknowledge constant encouragement and moral support rendered by Professor P.K. Saiprakash (Former Dean, Faculty of Science OU) and Professor T. Navaneeth Rao (Former Vice-Chancellor, OU; Principal, Nizam College and Head, Department of Chemistry, O.U).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnaiah, G., Rajanna, K.C., Reddy, K.R. et al. Cesium carbonate as efficient catalyst for chemoselective transesterification of β-ketoesters under conventional and unconventional conditions. Res Chem Intermed 41, 2739–2751 (2015). https://doi.org/10.1007/s11164-013-1383-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1383-x