Abstract
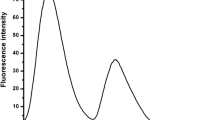
Schiff bases are widely investigated to develop novel chemosensors for selective identification and determination of anions. In this communication, we have taken an old but novel Schiff base, bis(5-nitrosalycilaldehyde)ethylenediamine (5-NO2-Salen, L), as a receptor to investigate the binding interaction with different anions such as F−, I−, Cl−, and Br− using spectrophotometric and computational approaches. Addition of anions accelerated the tautomerization process of L. The enolimine form of L interacts through the phenolic-OH by intermolecular hydrogen bond that intensified the color of the receptor, and a significant hyperchromic shift was observed due to the fluoride anion.







Similar content being viewed by others
References
P.A. Gale, Coord. Chem. Rev. 240, 191–221 (2003)
T. Gunnlaugsson, M. Glynn, G.M. Tocci, P.E. Kruger, F.M. Pfeffer, Coord. Chem. Rev. 250, 3094–3117 (2006)
A. Bianchi, K. Bowman-James, E. Garcia-Espana, Supramolecular Chemistry of Anions (Wiley, New York, 1997)
P.S. Dieng, C. Sirlin, Int. J. Mol. Sci. 11, 3334–3348 (2010)
G.V. Louie, P.D. Brownlie, R. Lambert, J.B. Cooper, T.L. Blundell, S.P. Wood, M.J. Warren, S.C. Woodcock, P.M. Jordan, Nature 359, 33–39 (1992)
L.H. Weinstein, A.W. Davison, Fluorides in the Environment (CABI Publishing, Cambridge, 2004)
K.J. Wallace, R.J Fagmemi, F.J. Folmer-Anderson, J. Morey, V.M. Lynth, E.V. Anslyn, Chem. Commun. 3886-3888 (2006)
S. Ayoob, A.K. Gupta, Crit. Rev. Environ. Sci. Technol. 36, 433–487 (2006)
C.D. Geddes, Meas. Sci. Technol. 12, R53–R88 (2001)
C. Suksai, T. Tuntulani, Chem. Soc. Rev. 32, 192–202 (2003)
B. Kuswandi, Nuriman, W. Verboom, D.N. Reinhoudt, Sensors 6, 978–1017 (2006)
C. Suksai, T. Tuntulani, Top. Curr. Chem. 255, 355–369 (2005)
S. Kubik, Chem. Soc. Rev. 38, 585–605 (2009)
M. Wenzel, J.R. Hiscock, P.A. Gale, Chem. Soc. Rev. 41, 480–520 (2012)
P. Dydio, D. Lichosyt, J. Jurczak, Chem. Soc. Rev. 40, 2971–2985 (2011)
Z. Xu, S.K. Kim, S.J. Han, C. Lee, G. Kociok-Kohn, T.D. James, J. Yoon, Eur. J. Org. Chem. 305, 8–3065 (2009)
Z. Xu, N.J. Singh, S.K. Kim, D.R. Spring, K.S. Kim, J. Yoon, Chem. Eur. J. 17, 1163–1170 (2011)
Y.M. Hijji, B. Barare, A.P. Kennedy, R. Butcher, Sens. Actuators B Chem. 136, 297–302 (2009)
Y. Zhou, J.Y. Jung, H.R. Jeon, Y. Kim, S.-J. Kim, J. Yoon, Org. Lett. 13, 2742–2745 (2011)
Y.-M. Zhang, Q. Lin, T.-B. Wei, D.-D. Wang, H. Yao, Y.-L. Wang, Sens. Actuators B Chem. 137, 447–455 (2009)
X. Bao, J. Yu, Y. Zhou, Sens. Actuators B Chem. 140, 467–472 (2009)
Q. Li, Y. Guo, J. Xu, S. Shao, Sens. Actuators B Chem. 158, 427–431 (2011)
A.K. Mahapatra, S.K. Manna, P. Sahoo, Talanta 85, 2673–2680 (2011)
V.K. Bhardwaj, M.S. Hundal, G. Hundal, Tetrahedron 65, 8556–8562 (2009)
L. Zang, D. Wei, S. Wang, S. Jiang, Tetrahedron 68, 636–641 (2012)
S. Dalapati, Md.A. Alam, S. Jana, N. Guchhait, J. Fluorine Chem. 132, 536–540 (2011)
X. Shang, J. Yuan, Y. Wang, J. Zhang, X. Xu, J. Mol. Struct. 1010, 52–58 (2012)
D. Sharma, R.K. Bera, S.K. Sahoo, Spectrochim. Acta. A 105, 477–482 (2013)
D. Sharma, A.R. Mistry, R.K. Bera, S.K. Sahoo, Supramol. Chem. 25, 212–220 (2013)
K.S. Murray, A.M. van den Bergen, B.O. West, Aust. J. Chem. 31, 203–207 (1978)
M.J. Frisch et. al., Gaussian 09, Revision A.1, (Gaussian Inc., Wallingford, 2009)
S.K. Sahoo, D. Sharma, R.K. Bera, J. Mol. Model. 18, 1993–2001 (2012)
D. Sharma, S.K. Sahoo, R.K. Bera, R. Kamal, J. Fluoresc. doi:10.1007/s10895-013-1178-x (2013)
Acknowledgments
The authors thank the Director, S V National Institute of Technology, Surat, for encouragement and for providing necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattiwala, N.M., Kamal, R. & Sahoo, S.K. Schiff base bis(5-nitrosalycilaldehyde)ethylenediamine as colorimetric sensor for fluoride. Res Chem Intermed 41, 391–400 (2015). https://doi.org/10.1007/s11164-013-1200-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1200-6