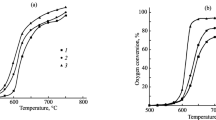
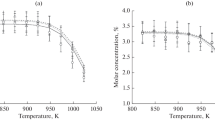
Abstract
Homogeneous gas-phase pyrolysis of propane was performed by using continuous CO2 laser irradiation for bulk heating of the reaction mixture. Laser energy was absorbed by ethylene, the main product of propane dehydrogenation, and transferred to the reaction medium via collisional relaxation. A mechanism of propane dehydrogenation is suggested to describe the pyrolysis process. The mechanism involves autocatalysis by ethylene and includes propane–ethylene interaction with the formation of ethyl and propyl radicals.






Similar content being viewed by others
References
N.N. Semenov, Some Problems of Chemical Kinetics and Reactivity (Pergamon Press, London, 1958), p. 59
F.O. Rice, K.F. Herzfeld, J. Am. Chem. Soc. 56, 284 (1934)
M.M. Papic, K.J. Laidler, Can. J. Chem. 49, 535 (1971)
A. Lifshitz, M. Frenclach, J. Phys. Chem. 79(7), 686 (1975)
A.M. Starik, N.S. Titova, L.S. Yanovskii, Kinet. Catal. 40(1), 7 (1999)
M.G. Ktalkherman, High Temp. 47, 5 (2009)
M.E. Dente, E.M. Ranzi, In Pyrolysis: Theory and Industrial Practice, vol. 133 ed. by L.F. Albriht, B.L. Crynes (Academic Press, New York, 1983)
A.S. Tomlin, M.J. Pilling, J.H. Merkin, J. Brindley, N. Burgess, A. Gough, Ind. Eng. Chem. Res. 34, 3749 (1995)
YuM Zhorov, Kinetika promyshlennykh organicheskikh reaktsii: Spravochnoe izdanie (Kinetics of Industrial Organic Reactions: A Handbook) (Khimiya, Moscow, 1989)
T.N. Mukhina, N.L. Barabanov, S.E. Babash et al., Piroliz uglevodorodnogo syr’ya (Pyrolysis of Hydrocarbon Stocks) (Khimiya, Moscow, 1987)
D.L. Allara, D. Edelson, Int. J. Chem. Kinet. 7, 479 (1975)
S.K. Layokun, D.H. Slater, Ind. Eng. Chem. Process Des. Dev. 18(2), 232 (1979)
A. Dombi, P. Huhn, Int. J. Chem. Kinet. 18, 227 (1986)
A. Dombi, I. Horvath, P. Huhn, Int. J. Chem. Kinet. 18, 255 (1986)
V.N. Snytnikov, T.I. Mishchenko, VlN Snytnikov, S.E. Malykhin, V.I. Avdeev, V.N. Parmon, Res. Chem. Intermed. 38(3), 1133 (2012)
V.N. Snytnikov, T.I. Mischenko, Vl.N. Snytnikov, O.P. Stoyanovskaya, V.N. Parmon, Kinet. Catal. 51(1), 10–17 (2010). doi:10.1134/S0023158410010039
V.A. Vshivkov, O.P. Sklyar, V.N. Snytnikov, I.G. Chernykh, Vychislitelnye Tekhnologii. 11(1), 35 (2006)
V.A. Vshivkov, O.P. Stoyanovskaya, Vychislitelnye Tekhnologii. 12(4), 42 (2007)
E. Hairer, G. Wanner, Solving Ordinary Differential Equations II: Stiff and Differential. Springer Series in Computational Mathematics (Springer, 2010), p. 614, ISBN:9783642052200
C.K. Westbrook, W.J. Pitz, Combust. Sci. Technol. 37, 117 (1984)
J.A. Manion, R.E. Huie, R.D. Levin, D.R. Burgess Jr., V.L. Orkin, W. Tsang, W.S. McGivern, J.W. Hudgens, V.D. Knyazev, D.B. Atkinson, E. Chai, A.M. Tereza, C.Y. Lin, T.C. Allison, W.G. Mallard, F. Westley, J.T. Herron, R.F. Hampson, D.H. Frizzell, NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Version 7.0 (Web Version), Release 1.4.3, Data version 2008.12, National Institute of Standards and Technology, Gaithersburg. http://kinetics.nist.gov/. Retrieved September 12, 2011
C.N. Hinshelwood, Proc. R. SOC. London Ser A 234, 301 (1956)
M.C. Lin, K.J. Laidler, Can. J. Chem. 44, 2927 (1966)
H.J. Curran, Int. J. Chem. Kinet. 38, 250 (2006)
D.M. Matheu, J.M. Grenda, J. Phys. Chem. 109, 5343 (2005)
S.P. Krishtal, A.M. Mebel, R.I. Kaiser, J. Phys. Chem. A 113, 11112 (2009)
T. Koike, W.C. Gardlner, J. Phys. Chem. 84, 2005 (1980)
Acknowledgments
This work has been supported by RFBR 12-08-00871, Russian Federation President Grant for the Leading Scientific Schools for funding (NSh 524.2012.3), Project UNIHEAT of Skolkovo Foundation, Integration Project no. 130, Federal Program "Scientific and Scientifical-Pedagogical cadres innovation Russia for 2009-2013" of the Federal Agency For Science And Innovation, RFBR grant no. 12-07-00065. The authors are grateful to Dr S.E. Malykhin for scientific and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snytnikov, V.N., Mishchenko, T.I., Snytnikov, V.N. et al. Autocatalytic dehydrogenation of propane. Res Chem Intermed 40, 345–356 (2014). https://doi.org/10.1007/s11164-012-0967-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0967-1