Abstract
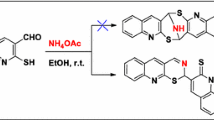
The 2,5-dihydroxy-1,4-benzoquinone-assisted oxy-Michael addition reaction between an aromatic aldehyde, EtOH and 2-naphthol or 6-hydroxyquinoline in the presence of HCl to afford the 1-[ethoxy(aryl)methyl]-2-naphthol and 5-[ethoxy(phenyl)methyl]-6-hydroxyquinoline derivatives at room temperature is described.
Graphical abstract





Similar content being viewed by others
References
M. Schapira, R. Abagyan, M. Totrov, J. Med. Chem. 46, 3045 (2003)
A.C. Goudie, L.M. Gaster, A.W. Lake, C.J. Rose, P.C. Freeman, B.O. Hughes, D. Miller, J. Med. Chem. 21, 1260 (1978)
P. Hurwitz, J.M. Thompson, Arch. Ophthal. 43, 712 (1950)
T.E. Archer, J.D. Stokes, J. Agric. Food Chem. 28, 877 (1980)
A.A. Kyle, M.V. Dahl, Am. J. Clin. Dermatol. 5, 443 (2004)
H.J. Roth, H. Fenner, Arzneistoffe, 3rd edn. (Deutscher Apotheker Verlag, Stuttgart, 2000), pp. 51–114
B. Duperray, M. Chastrette, M.C. Makabeh, H. Pacheco, Eur. J. Med. Chem. 11, 433 (1976)
J. Polanski, H. Niedbala, R. Musiol, B. Podeszwa, D. Tabak, A. Palka, A. Mencel, J.F. Mouscadet, M. Le Bret, Lett. Drugs Des. Disc. 4, 99 (2007)
L.Y. Vargas, M.V. Castelli, V.V. Kouznetsov, J.M. Urbina, S.N. Lopez, M. Sortino, R.D. Enriz, J.C. Ribas, S. Zacchino, Bioorg. Med. Chem. 11, 1531 (2003)
B. Podeszwa, H. Niedbala, J. Polanski, R. Musiol, D. Tabak, J. Finster, K. Serafin, J. Wietrzyk, S. Boryczka, W. Mol, J. Jampilek, J. Dohnal, D. Kalinowski, D.R. Richardson, Bioorg. Med. Chem. Lett. 17, 6138 (2007)
J. Berson, in The Chemistry of the Quinonoid Compounds, vol. 2, Part 1, eds. by S. Patai, Z. Rappoport (Wiley, Chichester, 1988), p. 455
P.G. Bulger, S.K. Bagal, R. Marquez, Nat. Prod. Rep. 25, 254 (2008)
Lignins, Occurrence, Formation, Structure and Reactions, eds. by K.V. Sarkanen, C. Ludving (Wiley, New York, 1971)
X. Weng, L. Ren, L. Weng, J. Huang, S. Zhu, X. Zhou, L. Weng, Angew. Chem. Int. Ed. Engl. 46, 8020 (2007)
E.E. Weinert, R. Dondi, S. Colloredo-Melz, K.N. Frankenfield, C.H. Mitchell, M. Freccero, S.E. Rokita, J. Am. Chem. Soc. 128, 11940 (2006)
M. Tomasz, A. Das, K.S. Tang, M.G.I. Ford, A. Minnock, S. Musser, M.J. Waring, J. Am. Chem. Soc. 120, 11581 (1998)
G. Gaudiano, M. Frigerio, P. Bravo, T.H. Koch, J. Am. Chem. Soc. 112, 6704 (1990)
E.A. Leo, J. Delgado, L.R. Domingo, A. Espinos, M.A. Miranda, R. Tormos, J. Org. Chem. 68, 9643 (2003)
Y. Chiang, A.J. Kresge, Y. Zhu, J. Am. Chem. Soc. 122, 9854 (2000)
L. Diao, C. Yang, P. Wan, J. Am. Chem. Soc. 117, 5369 (1995)
H. Wang, Y. Wang, K.L. Han, X.J. Peng, J. Org. Chem. 70, 4910 (2005)
H. Amouri, Synlett 10, 1357 (2011)
S. Arumugam, V.V. Popik, J. Am. Chem. Soc. 131, 11892 (2009)
M. Misra, R. Luthra, K.L. Singh, K. Sushil, in Comprehensive Natural Products Chemistry, vol. 4, eds. by D. H. R. Barton, K. Nakanishi, O. Meth-Cohn (Pergamon, Oxford, UK, 1999), p. 25
R. Mahrwald (ed.), Modern Aldol Reactions (Wiley, VCH, Weinheim, 2004)
B. Alcaide, P. Almendros, Eur. J. Org. Chem. 10, 1595 (2002)
A. Berkessel, H. Groerger, Asymmetric Organocatalysis (Wiley-VCH, Weinheim, 2005), pp. 71–73 and 79–80
C.F. Nising, S. Bräse, Chem. Soc. Rev. 37, 1218 (2008)
U.K. Ohnemüller, C.F. Nising, M. Nieger, S. Bräse, Eur. J. Org. Chem. 1535 (2006)
D.B. Ramachary, R. Mondal, Tetrahedron Lett. 47, 7689 (2006)
H. Li, J. Wang, T. E-Nunu, L. Zu, W. Jiang, S. Wei, W. Wang, Chem. Commun. 507 (2007)
D.R. Li, A. Murugan, J.R. Falck, J. Am. Chem. Soc. 130, 46 (2008)
H.L. Van Lingen, W. Zhuang, T. Hansen, F.P.J.T. Rutjes, K.A. Jogensen, Org. Biomol. Chem. 1, 1953 (2003)
T. Kito, K. Yoshinaga, S. Ohkami, K. Ikeda, M. Yamaye, J. Org. Chem. 50, 4628 (1985)
P. Bernal, J. Tamariz, Tetrahedron Lett. 47, 2905 (2006)
T. Kano, Y. Tanaka, K. Maruoka, Tetrahedron Lett. 47, 3039 (2006)
A. Shaabani, A.H. Rezayan, S. Keshipour, A. Sarvary, S.W. Ng, Org. Lett. 11, 3342 (2009)
A. Shaabani, A. Sarvary, A.H. Rezayan, S. Keshipour, Tetrahedron 65, 3492 (2009)
A. Shaabani, S. Keshipour, S. Shaabani, M. Mahyari, Tetrahedron Lett. 53, 1641 (2012)
S. Keshipour, S. Shojaei, A. Shaabani, Tetrahedron 68, 6141 (2012)
A. Shaabani, R. Ghadari, A. Sarvary, A.H. Rezayan, J. Org. Chem. 74, 4372 (2009)
A. Shaabani, A. Rahmati, E. Farhangi, Tetrahedron Lett. 48, 7291 (2007)
W. Su, D. Yang, C. Jin, B. Zhang, Tetrahedron Lett. 49, 3391 (2008)
Acknowledgment
We gratefully acknowledge financial support from the Research Council of the Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keshipour, S., Shaabani, A., Pedarpour, M. et al. An oxy-Michael addition: 2,5-dihydroxy-1,4-benzoquinone-assisted synthesis of 1-[ethoxy(phenyl)methyl]-2-naphthol and 5-[ethoxy(phenyl)methyl]-6-hydroxyquinoline derivatives. Res Chem Intermed 40, 149–156 (2014). https://doi.org/10.1007/s11164-012-0951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0951-9