Abstract
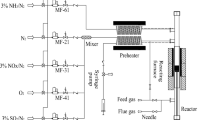
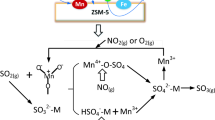
Direct synthesis of nanosheet Fe-ZSM-5 catalysts and their use for selective catalytic reduction (SCR) of NO x by ammonia were studied. XRD, BET, SEM, EPR, and NH3-TPD were used to understand the properties of catalysts with different iron loading. XRD confirmed the presence of the ZSM-5 crystal phase, and there was no Fe2O3 phase on the surface of the crystals. SEM showed the Fe-ZSM-5 catalysts comprised microspheres made up of nanosheets. EPR indicated that the iron was present as isolated Fe3+and FeO x oligomers uniformly dispersed throughout the crystals. NH3-TPD indicated that Fe-ZSM-5 (20,1:1) had maximum acid sites and density at approximately 250 and 450 °C, respectively. Fe-ZSM-5 (20,1:1) had the highest activity in the SCR reaction with NH3. It was also confirmed that Fe-ZSM-5 (20,1:1) had excellent resistance to SO2 and H2O under the SCR reaction conditions. The effects of water vapor and SO2, iron loading, and the Si/(Fe + Al) ratio were also investigated for these catalysts.









Similar content being viewed by others
References
H.Y. Huang, R.Q. Long, R.T. Yang, Appl. Catal. A 235, 241 (2002)
R.Q. Long, R.T. Yang, J. Catal. 201, 145 (2001)
K. Krishna, M. Makkee, Catal. Lett. 106, 183 (2006)
K. Krishna, G.B.F. Seijer, C.M.V.D. Bleek, M. Makkee, G. Mul, H.P.A. Calis, Catal. Lett. 86, 121 (2003)
H.Y. Chen, W.M.H. Sachtler, Catal. Today 42, 73 (1998)
R.Q. Long, R.T. Yang, J. Catal. 194, 80 (2000)
Z.G. Huang, Z.Y. Liu, X.L. Zhang, Q.Y. Liu, Appl. Catal. B 63, 260 (2006)
Y. Li, Q. Zhong, J. Hazard. Mater. 172, 635 (2009)
W.S. Kijlstra, J.C.M.L. Daamen, J.M.V.D. Graaf, B.V.D. Linden, E.K. Poels, A. Bliek, Appl. Catal. B 7, 337 (1996)
H.Y. Chen, X. Wang, M.H. Sachtler, Appl. Catal. B 194(195), 159 (2000)
R.Q. Long, R.T. Yang, J. Catal. 207, 224 (2002)
A.L. Kustov, T.W. Hansen, M. Kustova, C.H. Christensen, Appl. Catal. B 76, 311 (2007)
C.J.H. Jacobsen, C. Madsen, J. Houzvicka, I. Schmidt, A. Carlsson, J. Am. Chem. Soc. 122, 7116 (2000)
M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki, R. Ryoo, Nature 461, 246 (2009)
X. Feng, W.K. Hall, Catal. Lett. 41, 45 (1996)
X. Feng, W.K. Hall, J. Catal. 166, 368 (1997)
W.K. Hall, X. Feng, J. Dumesic, R. Watwe, Catal. Lett. 52, 13 (1998)
H.S. Cho, R. Ryoo, Micropor. Mesopor. Mater. 151, 107 (2012)
M. Iwasaki, K. Yamazaki, K. Banno, H. Shinjoh, J. Catal. 260, 205 (2008)
B.M. Reddy, K.N. Rao, G.K. Reddy, A. Khan, S.E. Park, J. Phys. Chem. C 111, 18751 (2007)
M.S. Kumar, M. Schwidder, W. Grünert, U. Bentrup, A. Brüchner, J. Catal. 239, 173 (2006)
A.V. Kucherov, M. Shelef, J. Catal. 195, 106 (2000)
T. Miyamoto, N. Katada, J.H. Kim, M. Niwa, J. Phys. Chem. B 102, 6738 (1998)
Y. Ni, A. Sun, X. Wu, G. Hai, J. Hu, T. Li, G. Li, Micropor. Mesopor. Mater. 143, 435 (2011)
Y.H. Seo, E.A. Prasetyanto, N. Jiang, S.M. Oh, S.E. Park, Micropor. Mesopor. Mater. 128, 108 (2010)
A. Ates, C. Hardacre, A. Goguet, Appl. Catal. A 441–442, 30 (2012)
A.C. Akah, G. Nkeng, A.A. Garforth, Appl. Catal. B 74, 34 (2007)
R.Q. Long, R.T. Yang, J. Catal. 207, 158 (2002)
A.M. Volodin, G.M. Zhidomirov, K.A. Dubkov, E.J.M. Hensen, R.A. van Santen, Catal. Today 110, 247 (2005)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 51078185 and no. 51106076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Qu, H., Zhang, J. et al. Preparation of nanosheet Fe-ZSM-5 catalysts, and effect of Fe content on acidity, water, and sulfur resistance in the selective catalytic reduction of NO x by ammonia. Res Chem Intermed 39, 4109–4120 (2013). https://doi.org/10.1007/s11164-012-0927-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0927-9