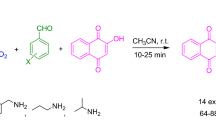
Abstract
Tetrabutyl ammonium bromide (TBAB) catalyzed one-pot synthesis of 4H-chromene via a three-component cyclocondensation of aryl aldehydes, cyanoacetate and dimedone in green media–water. Then, 4H-chromene reacted with acyl chloride in acetonitrile at refluxing with catalytic amount of DMAP to yield the title compounds in good yields. The structures of all title compounds were confirmed by 1H NMR, MS and elementary analyses. The X-ray crystallography of compound 3n indicated that there were strong intermolecular hydrogen bonds.
Graphical Abstract




Similar content being viewed by others
References
K. Singh, J. Singh, H. Singh, Tetrahedron 52, 14273–14280 (1996)
S. Hatokeyama, N. Ochi, H. Numata et al., J. Chem. Soc. Chem. Commun. 17, 1202–1204 (1988)
G.T. Brooks, A.P. Ottridge, R.C. Jennings et al., Pestic. Sci. 16, 571–588 (1985)
E.C. Witte, P. Neubert, A. Roesch, Ger. Offen. DE3427985, (1986)
N. Chand, W. Diamantis, R.D. Sofia, Brit. J. Pharmcol. 87, 443–448 (1986)
T. Hyana, H. Saimoto, Jpn Kokai Tokkyo Koho. JP 62181276, (1987)
V.B. Chavan, G.S. Sarate, N.S. Tankhiwale et al., Indian J. Anaesth. 42, 192–194 (1994)
Z.J. Liu, Z.R. Li, Chin. J. Org. Chem. 11, 433–436 (1991)
V.F. Paula, L.C.A. Barbosa, A.J. Demuner et al., Pest Manage. Sci. 56, 168–174 (2000)
J.S. Pizey, R.L. Wain, J. Sci. Food. Agric. 10, 577–584 (1959)
M. Suarez, E. Salfran, Y. Verdecia et al., Tetrahedron 58, 953–960 (2002)
Q.Y. Zhuang, N. Wu, D.Q. Shi et al., Chin. J. Org. Chem. 26, 1217–1220 (2006)
S.J. Tu, Y. Gao, C. Guo et al., Synth. Commun. 32, 2137–2141 (2002)
Z.Q. Jiang, S.J. Ji, J. Lu et al., Chin. J. Chem. 23, 1085–1089 (2005)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625–8627 (2004)
L.C. Rong, X.Y. Li, H.Y. Wang et al., Synth. Commun. 36, 2363–2369 (2006)
G. Kaupp, M.R. Naimi-Zamal, J. Schmeyers, Tetrahedron 59, 3753–3760 (2003)
C.J. Li, Chem. Rev. 93, 2023–2035 (1993)
A. Lubineau, Chem. Ind. (London) 4, 123–126 (1996)
A. Meijer, S. Otto, J.B. Engberts, J. Org. Chem. 63, 8989–8994 (1998)
R. Ballini, G. Bosica, Tetrahedron Lett. 37, 8027–8030 (1996)
R. Ballini, G. Bosica, J. Org. Chem. 62, 425–427 (1997)
F. Bigi, S. Carloni, L. Ferrari et al., Tetrahedron Lett. 42, 5203–5205 (2001)
G.M. Ziarani, A. Abbasi, A. Badiei et al., J. Chem. 8, 293–299 (2011)
S.B. Bandgar, B.P. Bandga, B.L. Korbad et al., Aust. J. Chem. 60, 305–307 (2007)
X.S. Wang, D.Q. Shi, S.J. Tu et al., Synth. Commun. 33, 119–126 (2003)
Q.Y. Wang, Master’s degree thesis of Hubei University, China, (2009)
S.J. Tu, H. Wang, J.Q. Feng et al., Synth. Commun. 31, 2663–2666 (2001)
S.J. Tu, C.B. Miao, Y. Gao et al., Chin. J. Chem. 20, 703–706 (2002)
D.Q. Shi, S. Zhang, Q.Y. Zhuang, Chin. J. Org. Chem. 25, 1570–1574 (2005)
G.M. Sheldrick, SHELXS-97: program for crystal structure refinement; Göttingen, (1997)
SAINT and SMART; Bruker AXS: Madison, (2003)
Acknowledgments
We are grateful to financial support from the National NSFC (No.21162007), Project for Department of Education, Hainan Province (No.Hj2009-19) and Hainan University Postdoctoral Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, Zg., Wang, Ly., Cao, Y. et al. Synthesis of 2-amide-3-carboxylate-4-aryl-4H-chromene derivatives. Res Chem Intermed 38, 1751–1760 (2012). https://doi.org/10.1007/s11164-012-0500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0500-6