Abstract
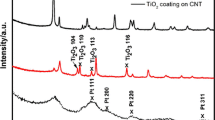
The electrochemical deposition of Pt nanoparticles on carbon nanotube (CNTs) supports and their catalytic activities for an electro-oxidation were investigated. Pt catalysts of 4–12 nm average crystalline size were grown on supports by changing applied potential methods such as sweep-potential or step-potential. Electroplating of 24-min time by a step-applied potential was enough to obtain small crystalline-size 4.6-nm particles, resulting in good electrochemical activity. The catalysts’ loading contents could be controlled by increasing the deposition time. The crystalline sizes and structures of the Pt/support catalysts were analyzed using X-ray diffraction (XRD). The electrochemical properties of the Pt/support catalysts were studied according to their characteristic current–potential curves in a methanol solution. As a result, the electrochemical activity was increased by enlarging the plating time. The activity reached the maximum at 24 min and then decreased. The enhanced electroactivity for catalysts by step-potential methods could be explained by the changes of the crystalline size and crystalline structures of the catalysts.





Similar content being viewed by others
References
C.Y. Chen, P. Yang, Y.S. Lee, K.F. Lin, J. Power Sources 141, 24 (2005)
A.S. Arico, S. Srinivasan, V. Antonucci, Fuel Cells 1, 133 (2001)
J.W. Guo, T.S. Zhao, J. Prabhuram, C.W. Wong, Electrochim. Acta 50, 1973 (2005)
A.S. Arico, P. Creti, E. Modica, G. Monforte, V. Baglio, V. Antonucci, Electrochim. Acta 45, 4319 (2000)
S. Kim, M.H. Cho, J.R. Lee, H.J. Ryu, S.J. Park, Korean Chem. Eng. Res. 43, 756 (2005)
T. Frelink, W. Visscher, J.A. Rvan, J. Electroanal. Chem. 382, 65 (1995)
V. Lordi, J. Yao, J. Wei, Chem. Mater. 13, 733 (2001)
K.H. Choi, H.S. Kim, T.H. Lee, J. Power Sources 75, 230 (1998)
C.A. Bessel, K. Laubernds, N.M. Rodriguez, R.T. Baker, J. Phys. Chem. 105, 1115 (2001)
T. Hyeon, S. Han, T.E. Sung, K.W. Park, Y.W. Kim, Angew. Chem. 42, 4357 (2003)
A.S. Arico, A.K. Shulka, K.M. Khatib, P. Creti, V. Antonucci, J. Appl. Electrochem. 29, 671 (1999)
S. Kim, S.J. Park, J. Power Sources 159, 42 (2006)
S. Kim, M.H. Cho, J.R. Lee, S.J. Park, J. Power Sources 159, 46 (2006)
S. Kim, S.J. Park, Electrochim. Acta 52, 3013 (2007)
E. Higuchi, K. Adachi, S. Nohara, H. Inoue, Res. Chem. Intermed. 35, 985 (2009)
H.M. Lee, S.Y. Park, K.T. Park, U. Jung, K. Chun, C.D. Woong, S.H. Kim, Res. Chem. Intermed. 34, 787 (2008)
S. Lim, D. Jung, S.-H. Yoon, I. Mochida, Carbon Letters 9, 47 (2008)
K. Kinoshita, Carbon: Electrochemical and Physicochemical Properties (John Wiley, New York, 1988), p. 31
Acknowledgments
This research was supported by the Converging Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093655) and Pusan National University Research Grant, 2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, SJ., Kim, S. Electrochemical properties of carbon nanotube-supported metallic catalysts prepared by changing a sweep- or step-applied potential. Res Chem Intermed 36, 693–701 (2010). https://doi.org/10.1007/s11164-010-0171-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-010-0171-0