Abstract
The common carp Cyprinus carpio is one of the most widely-distributed freshwater fishes in the world. Due to its value for conservation and fisheries in several native/translocated areas of distribution and its detrimental effects on the aquatic ecosystem in most invasive areas, robust age-based population dynamics models are required for successful management of this species. The present study provides a global review of age determination in carp, including a historical account of ageing methods, an assessment of the relative utility of ageing structures, and an evaluation of precision and accuracy (i.e. validation) of age estimates. Historically, scales were by far the most widely-employed structure, followed by the operculum, otolith, dorsal spine, vertebra and fin ray. However, in countries where carp is categorised as ‘high risk’ of impact, use of alternative structures to the scale was predominant. Causal criteria analysis showed scales and opercula to provide inconsistent evidence for successful annulus identification/counting, whereas consistent evidence was found for otoliths, dorsal spines, vertebrae and (pectoral) fin rays. Precision was always above reference thresholds for scales, whereas for otoliths, dorsal spines and fin rays was in several cases below. Accuracy was addressed sporadically and mostly in high-risk countries. It is suggested that dorsal spines or pectoral fin rays should be used in lieu of scales as non-lethal ageing structures, and otoliths (or vertebrae, pending more research) otherwise, and that validation should always be attempted as part of the set-up of more appropriate ageing protocols and use of correct terminology.
Similar content being viewed by others
Introduction
Age determination in fish is fundamental for the management of fisheries (e.g. Hilborn and Walters 2013) and for understanding species’ life histories and their population dynamics (Beddington and Kirkwood 2005). Various calcified structures (or ‘hard parts’), including scales, opercula, vertebrae, spines, fin rays and otoliths (Casselman 1983), are available for ageing fish, and these structures are sometimes used in conjunction for comparative purposes (e.g. Vilizzi and Walker 1999; Khan and Khan 2009). Age determination typically involves the counting of annual (or daily) increments under the assumption that these were formed on an equivalent temporal interval. This assumption is verified through validation, which is equivalent to determining the accuracy of an age estimate (Campana 2001).
Not surprisingly, failure to accomplish the aforementioned accuracy–precision requirements is likely to result in erroneous age estimates. This ultimately leads to biased understanding of fish population dynamics, with consequences for the management and conservation of species (e.g. Campana 2001; Crook et al. 2013). Hence, the importance of age validation (cf. Beamish and McFarlane 1983) for fish and fisheries biologists (sensu Balon 1999), including the set-up of so-called ‘production ageing’ programmes (i.e. reference collections of ageing structures and quality control monitoring), which have become an intrinsic component of modern fisheries laboratories in many parts of the world (Campana 2001).
Highly valued in most of its native areas of distribution (Vilizzi 2012), the common carp Cyprinus carpio L. (hereafter, ‘carp’) is one of the most widely-distributed freshwater fishes in the world (Welcomme 1988; Casal 2006) due to introductions for farming and aquaculture that began in Roman times (Balon 1995; Copp et al. 2005). However, in many of its non-native areas the carp is regarded as an invasive (pest) species, with documented and often severe adverse impacts on freshwater ecosystems (Vilizzi and Tarkan 2015; Vilizzi et al. 2015). As one of the most studied freshwater fish species, both by historical and geographical extent, it is therefore not surprising that a vast amount of information has been generated on (amongst other ecological aspects) its age and growth (Vilizzi and Copp 2017). At the same time, population dynamics models have recently been developed for the management of carp in heavily-invaded regions of North America and Australasia (Brown and Walker 2004; Lechelt and Bajer 2016; Weber et al. 2011, 2016; Koehn et al. 2017). Since parameterisation of these models requires estimated ages and/or age-based growth parameters (e.g. von Bertalanffy growth function: VBGF), the availability of precise and accurate age estimates from reliable ageing structures is of paramount importance.
The present study provides a historical account of age determination in carp and evaluates the relative utility, precision and accuracy of the various ageing structures used. The specific objectives are to: (1) provide a historical evaluation (cf. ‘narrative’ review: sensu Webb et al. 2013) of the use and effectiveness of a range of ageing structures for carp; (2) assess the relative utility of these ageing structures through a ‘systematic’ review (sensu Webb et al. 2013) by means of an evidence-based, causal criteria analytical approach; and (3) evaluate the precision and accuracy (validation) of age estimates in carp. In light of the existing information, recommendations are made as to which ageing structure(s) should be routinely employed for ageing carp and how quality control programmes could be implemented. The ultimate aim is to assist fish biologists and fisheries managers to refine age and growth based population dynamics models for improved management of this important species.
Methodology
History
The literature material surveyed consisted of published sources, including peer-reviewed papers, theses, dissertations and, occasionally, reports. The criteria for inclusion of a study into the review were that it should: (1) have been carried out on carp living under natural conditions (i.e. in the wild); (2) provide mean length-at-age values and/or corresponding VBGF parameter estimates for the carp population(s) under investigation; and (3) ‘explicitly’ mention the structure(s) used for ageing (e.g. reference to ‘standard ageing protocols’, albeit most likely scale-based, was not accepted as sufficient evidence). These literature sources were then complemented with those studies investigating the morphology, physiology and processing techniques of the various structures used/attempted for ageing carp, with special emphasis on the otolith.
For each age-growth study selected as per above, the country of origin of the study and the distributional range of carp were noted. The species’ areas of distribution were distinguished into native, native/translocated and introduced. As such, non-native carp populations were those either introduced or translocated into water bodies located outside the wild form’s distributional range. This was based on the distributional map of the Eurasian landmass for wild carp provided by Kirpichnikov (1999; see also Chistiakov and Voronova (2009), noting that several countries span the carp’s native, translocated and introduced ranges. Within the introduced range, countries were categorised as either ‘medium risk’ or ‘high risk’ relative to the presence of carp following Vilizzi et al. (2015), and those countries not originally included in that study were assessed based on additional evidence (i.e. Colombia: Zambrano et al. 2006; Greece: Perdikaris et al. 2016; Portugal: Almeida et al. 2013; South Africa: Marr et al. 2017).
Relative utility
The Eco Evidence approach (Norris et al. 2012; Webb et al. 2013), which is a form of causal criteria analysis adapted to the environmental sciences, was used for weighting and combining evidence from those studies investigating the relative utility of the ageing structures. The studies included were those dealing with age and growth that relied on a ‘suite’ of ageing structures as well as those specifically focused on the comparative aspects of such structures (notably, studies only qualitatively assessing the utility of a certain structure, i.e. without enumerating annuli, were not included). The Eco Evidence framework consists of eight steps (Table 1): in step 1, the utility of the six most widely-used ageing structures plus the eye lens (because of one comparative study including it) to age carp successfully was assessed; in step 2, the ‘context’ was defined as the ageing of carp at the global (worldwide) level; in step 3, the conceptual model assumed that each structure would allow to age carp successfully by the correct identification of annuli; in step 4, the conceptual model was exemplified by seven corresponding hypotheses (i.e. one for each ageing structure evaluated) of successful annulus identification/counting on the structure used; in step 5, the evidence provided in support consisted of the reviewed comparative studies; and in step 7 (note that step 6 was not necessary), given that the framework was originally designed for the evaluation of ‘natural experiments’ for the monitoring of ecological impacts, a ‘best-match’ approach was employed. To this end, following the weigthing system of Norris et al. (2012), for those studies that assessed the utility of a specific structure relative to other reference structure(s), a ‘reference/control versus impact (no before)’ design was matched that provides a weight of 2, with one control structure contributing an additional weight of 2, more than one control structure a weight of 3, and with the reference structure (only one in all cases) providing a weight of 0. Whereas, for those studies that evaluated a suite of structures simultaneously, a ‘gradient response model’ design was matched that provides a weight of 0, with up to three structures contributing also a weight of 0, four structures a weight of 2, and five structures a weight of 4.
The desktop Eco Evidence v1.1.1 analyser software (Nichols et al. 2011) was used for implementation of causal criteria analysis. Each ‘evidence item’ (=assessment of a certain structure) was assigned an overall (i.e. summation) evidence weight based on the weighting system defined above. For each of the seven structure-related hypotheses, the resulting weights were then summed for all evidence in favour of the hypothesis and for all evidence against the hypothesis. Following Norris et al. (2012), the sums were then compared to a threshold value of 20 points, which has proven satisfactory in previous applications of the methodology (Webb et al. 2013; Vilizzi et al. 2015). This resulted in one of four conclusions for each hypothesis (i.e. ‘support for hypothesis’: ≥ 20 in favour, < 20 against; ‘inconsistent evidence’: ≥ 20 in favour, ≥ 20 against; ‘insufficient evidence’: < 20 in favour, < 20 against; ‘support for alternative hypothesis’: < 20 in favour, ≥ 20 against). Importantly, to assess the level of support for the seven hypotheses, and therefore pass judgement (step 8 of the framework), the ‘quantifiable effect’ equated in all cases to successful (or unsuccessful) identification of annuli by using a certain ageing structure.
Precision and accuracy
The age-growth literature on carp was screened for studies that assessed the precision of ageing structures. Precision was evaluated both within interpreter and between interpreters, as well as between ageing structures. The two most widely-employed indices for measuring precision, i.e. the average percentage error (APE) and the coefficient of variation (CV), were used in the comparisons (see Campana 2001). Since both indices are considered to be superior to the percentage agreement (Campana 2001), albeit reported in some studies, this was not included in the review. As (empirical) target levels of desirable precision, the values of 5.5% for the APE and of 7.6 or 5% for the CV (Campana 2001) were used as thresholds for all comparisons.
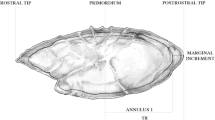
Similar to precision, the literature was screened for evidence of an evaluation of accuracy (validation). In some cases, this was the topic of a dedicated study, which was employed by the authors in support to one (or more) other related studies in which age and growth in carp were assessed. Validation methods were categorised as: (1) release of known age and marked fish; (2) mark-recapture of chemically-tagged fish; (3) discrete length modes sampled for age structures; and (4) marginal increment analysis (MIA) (terminology after Campana 2001). Finally, for each study both the structure(s) for which validation was accomplished and the location (country) where the study was conducted were noted.
Results
History
In total, 167 age-growth studies on carp spanning nearly 90 years provided information on the type of ageing structure(s) used (Table S1 in Supporting Information). Scales were by far the most commonly employed, followed (in decreasing order) by the operculum, otolith, dorsal spine, vertebra and fin ray (pectoral or anal) (Fig. 1a). However, unlike scales, which represented nearly 76% of the total proportion of studies, use of the other structures was always < 10%. Scales were the only structure employed before the 1950s, and accounted for the largest proportion of studies in all decades from the 1950s onwards, and in the 2010s still represented 63% of the total studies (Fig. 1b). Use of opercula and dorsal spines started in the 1950s, of vertebrae in the 1960s, of otoliths in the 1980s, and of fin rays in the 1990s. Scales were the only structure employed for ageing carp in its native range, and were by far the predominant structure used in its native/translocated range, with only China and Turkey relying also on fin rays, opercula and vertebrae, respectively as alternative structures (Fig. 2). Whereas, the spectrum of structures, other than scales, used to age carp in its introduced range was broad and consisted of opercula, otoliths, dorsal spines, vertebrae, fin rays. This was especially in countries where carp was categorised as ‘high-risk’, e.g. South Africa and Australia, where scales were used in only 20 and 25% of the reviewed studies, respectively. An in-depth, ageing structure-wise historical description is provided below (see also Fig. 3).
Proportion of studies using one of the six most common structures for ageing carp according to country (including total number of populations aged). Countries are grouped based on carp’s origin (see text for explanation), with ‘High’ and ‘Medium’ indicating the risk level of introduced-origin countries based on Vilizzi et al.’s (2015) categorisation (risk level of countries marked by an asterisk evaluated in the present study)
Scale
The age of cyprinid fishes has traditionally been estimated from scales (e.g. Jearld 1983; Mann 1991), with the identification of increment (annulus) patterns in carp dating back as far as the late 1700s (van Leeuwenhoek 1798; see also Jackson et al. 2007). However, it was at the turn of the nineteenth century that lepidological studies (i.e. investigating the morphology and characteristics of fish scales) contributed to the establishment of more rigorous protocols for annulus identification on this ageing structure (Hoffbauer 1898, 1905; see also Van Oosten 1928). Several age-growth studies have provided descriptions of the morphology of carp scales, with emphasis on the arrangement, pattern and (time of) formation of both annuli and circuli (i.e. thinner growth lines, including their ‘crossing-over’ or ‘anastomosis’), as well as related criteria for identification (Oliva 1955; Balon 1957; Vostradovský 1962; Das and Fotedar 1965; Effendie 1968; Jones 1974; Deng et al. 1981; Wang 1983; Johal et al. 1984). Also available are ‘dedicated’ lepidological studies that investigated: time of annulus formation (Frey 1942); morphology and arrangement of so-called ‘radii’ (Matsui 1949); criteria for identification of ‘true’ and ‘false’ annuli (Talaat and Oláh 1986); early developmental stages of scale formation (El-Fiky 1993); genetics of scale pattern formation (Casas et al. 2013); arrangement of scales (in mirror carp: see below) as a natural marker for individual fish identification (Huntingford et al. 2013); and the ultrastructure of the focal region of regenerated scales as an alternative to the use of molecular markers for identification of (invasive) carp populations (Johal et al. 2014).
The scales of mirror carp (the scalation variant genetically referred to as ‘scattered’ (Balon 1995; Kirpichnikov 1999) have also received attention in age determination studies. Das and Fotedar (1965) were the first to estimate the age of mirror carp successfully—they also contributed a detailed description of annulus patterns and criteria for their identification. Similarly, in all other age-growth studies of mirror carp (mainly carried out in Anatolia, Turkey: Sarıhan 1980; Akyurt 1987; Karakoç and Sarıhan 1987; Okumuş and Tekelioğlu 1987; Çetinkaya et al. 1995a, b; Kırankaya and Ekmekçi 2004; Kırankaya 2007; but see Prochelle and Campos 1985), no difficulties in annulus identification have seemingly been reported (but see Gümüş 1998). This is contrary to other studies, which have pointed to a total lack of interpretability of scales in this carp scalation variant (Linfield 1982; Vilizzi 1997).
Despite the predominant use of scales for ageing carp, several drawbacks with the interpretation of these structures have been encountered, including: (1) crowding of annuli towards the edge in older fish, (2) supernumerary rings (cf. ‘spawning checks’ and ‘pseudo-annuli’), (3) re-sorption, (4) lack of deposition of the first annulus, and (5) difficulties in locating the first annulus (Krumholz 1956; Christenson and Smith 1965; Starrett and Fritz 1965; Marlborough 1967; Linfield 1982; Lubinski et al. 1984; Fernández-Delgado 1990). On the other hand, the advantages with using scales include: (1) their ease of collection and preparation, and (2) the possibility for mark-recapture studies (i.e. scale removal need not harm the fish).
Operculum
The operculum has proven a valid complement or alternative to scales in carp age-growth studies. This structure has generally been used in conjunction with either scales only (English 1952; McConnell 1952; Rehder 1959; Jester 1974; Çetinkaya 1992; Çetinkaya et al. 1995a, b; Şen 2001; Tempero et al. 2006) or scales plus other hard parts (Effendie 1968; Lubinski et al. 1984; Cochrane 1985; Raina 1987; Bhandari et al. 1993; Vilizzi and Walker 1999), and seldom on its own (Tsimenide 1978; Çolakoğlu and Akyurt 2011) (Table S1 in Supporting Information).
Ridge-like projections radiating from the fulcrum (referred to as ‘buttresses’ or ‘fingers of bone’: English 1952 and McConnell 1952, respectively) may often obscure the first one to three annuli, challenging in such cases correct age estimation. The relative ease of collection of the operculum (albeit involving the sacrifice of the fish) is offset by the time required to prepare it for examination. In this respect, conventional processing methods (i.e. boiling to allow skin separation and/or scrubbing with a stiff brush) may prove time consuming compared to the removal and preparation of scales (e.g. Jones 1974).
Otolith
The unique chronological properties of otoliths relative to other calcified structures for ageing fish are widely known, and these typically relate to both annulus and micro-increment counting but also to micro-chemistry examination (Campana and Thorrold 2001). The anatomical differences in the vestibular apparatus of (typical) non-ostariophysan versus ostariophysan (cyprinoid) teleost fishes have been described (Secor et al. 1991), and specifically for carp (Li et al. 2009). Briefly, in non-ostariophysan teleost fishes, the sagitta (saccular aragonitic otolith) is routinely used for annulus identification and the lapillus (utricular aragonitic otolith) for micro-increment identification, whereas the third pair of otoliths, namely the vateritic lagenar asterisci, is not generally employed. On the contrary, in ostariophysans (including carp), the asteriscus has proven useful for reliable annulus counts (see below), unlike the inconspicuous, needle-shaped sagitta. Whereas, similar to the other teleost fishes, the lapillus is useful for micro-increment enumeration (see below and Fig. 3).
In carp, the use of otoliths for their chronological properties (including ageing) has been fraught with difficulties and terminological inconsistencies (Table 2). The first attempts to use otoliths for ageing carp failed as a result of what was reported as ‘small size’ of the pair examined (Jones 1974; Wichers 1976; Hume et al. 1983)—a statement that most likely points to the examination of the lapilli (see also Bishai and Labib 1978). Only starting from the mid-1980s were otoliths successfully used to age carp (Lubinski et al. 1984; Raina 1987; Pinilla et al. 1992), and these were most likely the asterisci, which in one study were referred to as the ‘largest’ pair of otoliths but erroneously called ‘sagittae’ (Lubinski et al. 1984). However, it was not until the mid-1990s that the peculiarities of carp otoliths were finally clarified (Vilizzi and Walker 1995), indicating the usefulness of the asterisci for annulus identification (Vilizzi and Walker 1998, 1999; Vilizzi et al. 1998) and of the lapilli for microincrement counts (Vilizzi 1998). These studies have since paved the way for: (1) refinement of validation of annulus counts on asterisci (Brown et al. 2004; Winker et al. 2010) and of micro-increment counts on lapilli (Smith and Walker 2003); (2) implementation of related age-growth studies on both adult (Diggle et al. 2004; Brown et al. 2005; Coulter et al. 2008; Bajer et al. 2009; Winker et al. 2011; Colvin et al. 2012; Hutchison et al. 2012; Amouei et al. 2013) and 0 + carp (Gilligan and Schiller 2003; Diggle et al. 2004; Phelps 2006; Britton et al. 2007; Phelps et al. 2008; Hutchison et al. 2012); (3) ageing structure comparisons (Yılmaz and Polat 2008; Yates et al. 2016); and (4) biometrical studies of otolith to body length relationships (Bostanci 2009). However, some other studies have still used the incorrect terminology (Gümüş 1998; Diggle et al. 2004; Amouei et al. 2013), or omitted explicit mention of the pair used (Temizer and Şen 2008; Aydin et al. 2009; Omar and Amohamed 2016).
Unlike the lapilli, which require sectioning for micro-increment counts (except in larvae with < 10–30 micro-increments: Vilizzi 1998; Gilligan and Schiller 2003), annulus counts can be made either on whole or sectioned asterisci (Vilizzi and Walker 1999). However, the majority of age-growth studies has relied on the sectioning method (Diggle et al. 2004; Brown et al. 2005; Coulter et al. 2008; Bajer et al. 2009; Winker et al. 2011; Colvin et al. 2012; Hutchison et al. 2012; Amouei et al. 2013), and only two of them have examined whole asterisci (Winker et al. 2011). Whereas, the ‘broken-and-burnt’ method (www.afsc.noaa.gov/refm/age/procedures.htm; accessed 30/12/2017) has so far been attempted for ageing structure comparisons only (Aydin et al. 2009) (Table 2). Finally, in the majority of age-growth studies, otoliths have been used as the only ageing structure (Pinilla et al. 1992; Vilizzi and Walker 1998; Brown et al. 2003, 2005; Diggle et al. 2004; Coulter et al. 2008; Bajer et al. 2009; Winker et al. 2011), and less frequently in association with other hard parts (Colvin et al. 2012; Lubinski et al. 1984; Raina 1987; Vilizzi and Walker 1999) (Table S1 in Supporting Information).
Although not strictly related to ageing, the use of otoliths for micro-chemistry studies in carp emphasises the importance of the correct identification of the pair employed (cf. Vilizzi and Walker 1995). Thus, studies have used either the ‘larger’ asterisci (Crook and Gillanders 2006; Blair 2008; Li et al. 2011; Blair and Hicks 2012) or the lapilli (Macdonald et al. 2010; Crook et al. 2013; Macdonald and Crook 2014), even though remarkable differences in microchemistry have been observed between the two pairs, suggesting the need for their concomitant employment (Macdonald et al. 2012).
The process of otolith extraction (i.e. of asterisci and, especially, lapilli) in carp, apart from being ‘destructive’, can be fairly involved relative to non-ostariophysan fishes (Vilizzi and Walker 1999; Macdonald et al. 2012), and if the sectioning method is being used, then further processing time is required (Vilizzi and Walker 1999; Brown et al. 2005). In a comparative study of whole and sectioned asterisci (Vilizzi and Walker 1999), no substantial advantage was found in annulus identification using the sectioning method. This finding has been indirectly corroborated by the ability to identify clearly biannual increment deposition on whole asterisci, with sections discarded because of poor interpretability (Winker et al. 2010).
Dorsal spine
In carp, the dorsal spine consists of a large bone with a strongly-serrated posterior edge (Bănărescu 1964). Although its first documented use for ageing carp dates back to the mid-1960s (Carlton and Jackson 1964; Starrett and Fritz 1965), previous studies had already referred to ‘standard methods’ of scale and spine analysis (Schoonover and Thompson 1954; Jackson 1955; Sandoz 1960). A more recent experimental study using fluorescent markers demonstrated the formation of so-called lignes d’arrêt de croissance (Meunier and Pascal 1981/1982), which provided a physiological basis for their use in a follow-up assessment of the growth of an individual cohort of carp (Jestin et al. 1985). The dorsal spine has been used either as the sole ageing structure (Wichers 1976; Katzenmeyer 2010; Weber et al. 2015) or, more often, in conjunction with others (Schoonover and Thompson 1954; Jackson 1955; Sandoz 1960; Starrett and Fritz 1965; Lubinski et al. 1984; Colvin et al. 2012) (Table S1 in Supporting Information).
Different sectioning planes along the length of the dorsal spine have been shown to result in deviations in back-calculated growth from annuli (Wichers 1976). This issue was recently addressed in a study showing that sections taken at ≤ 25% of the length of the dorsal spine would provide the most precise age estimates. As a non-lethal method, the use of this structure to estimate age in carp has proved a valid alternative to scales, even though more time is required for preparation and sectioning compared to the latter (Jearld 1983).
Vertebra
Vertebrae have been used traditionally to age elasmobranchs (Jearld 1983), but also carp, with the first documented report being from the late 1960s (Effendie 1968). Vertebrae have since been used in conjunction with other structures (Cochrane 1985; Bhandari et al. 1993). The most detailed description of the vertebra-based ageing method in carp is from a study on this species under farming conditions, which showed successful age determination in a sub-tropical climate (Bishai and Labib 1978). More recently, the only two age-growth studies relying exclusively on vertebrae (Yılmaz et al. 2012; Yüce et al. 2016) seem to have drawn from comparative studies pointing to the higher reliability of vertebrae (Temizer and Şen 2008; Yılmaz and Polat 2008) (Table S1 in Supporting Information). Vertebrae from the anterior part of the dorsal spine have been used, with the third (Bishai and Labib 1978) or 7th and 8th (Bhandari et al. 1993) having proved more reliable in some cases.
Fin ray
The use of fin rays as a reliable method for age determination for fishes including carp was demonstrated long ago (Boyko 1950). However, use of these structures in carp age-growth studies remains limited, and is found either in conjunction with scales (Liang et al. 1993) or, more recently, alone (Weber et al. 2010)—in the latter case based upon findings on their reliability and precision (Phelps et al. 2007, 2008) (Table S1 in Supporting Information). Similar to the dorsal spine, the use of fin rays does not require sacrificing the fish and, in general, appears to be a good alternative to scales (cf. Beamish 1981).
Other ageing methods
Although not a calcified structure, the weight of the eye lens has been proposed as an alternative method for ageing carp (Carlton and Jackson 1968). However, overlap in eye lens weight ranges between older age classes may represent a limitation on the use of this method (Crivelli 1980). No age-growth studies on carp populations have so far employed the eye lens.
Finally, the use of telomere length has recently been investigated as an alternative (non-lethal) method to increment-based age estimations on hard parts (Izzo et al. 2014). Telomeres are nucleotide and protein complexes located at the ends of vertebrate chromosomes. Findings from the only study so far investigating this method reported a significant increase in telomere length with increasing fork length, but also reported limitations due to poor distinction of individual age classes.
Relative utility
In total, 26 studies were found in which a comparative assessment of two or more ageing structures was provided (Table 3). Not surprisingly, the majority of assessments involved scales, followed by opercula, otoliths and dorsal spines, and vertebrae and fin rays (plus one assessment on the eye lens). Causal criteria analysis indicated inconsistent evidence for the usefulness of the scale and operculum, but supported the hypothesis of successsful annulus identification/counting for otoliths, dorsal spines, vertebrae and fin rays—but insufficient evidence for the eyes lens, due to only one comparative study being available (Table 4). Notably, two studies (i.e. Jones 1974; Hume et al. 1983) pointing to the limitations of some hard parts other than scales were not included into the systematic review because their findings were of qualitative value only (i.e. no evaluation or counting of annuli).
Precision and accuracy
Precision was evaluated by eleven studies in total, which analysed the scale, operculum, otolith, dorsal spine, (pectoral) fin ray and vertebra (Table 5). Within interpreters, the mean APE was lowest for the otolith, followed by the scale, operculum and dorsal spine; between interpreters, this index was again lowest for the otolith, followed by the operculum and scale (no data were available for the dorsal spine). Within interpreters, the mean CV was lowest for the otolith, followed by the operculum, scale and dorsal spine (one study only); whereas between interpreters, this index was lowest for the dorsal spine, followed by the fin ray, otolith, operculum and scale. Values below the 5.5% threshold for the APE were achieved for the operculum (one study out of three) and the otolith (three studies out of seven; but also for the only available assessment on the vertebra); below 5% for the CV were achieved for the dorsal spine (two studies out of four) and the fin ray (one study out of two); and below 7.6% for the CV (but higher than 5%) were achieved for the operculum (one study out of four), the otolith (three studies out of five), and the dorsal spine (two studies out of four)—noting that in the latter case all four studies assessing between-interpreter precision by the CV fell below the desirable levels of precision. Across studies, the mean APE was always above threshold, whereas the (between-interpreter) CV was below the higher threshold for the dorsal spine and fin ray. There were in total eight between-structure comparisons for precision, which was assessed by the APE and/or CV (Table 6). The range in index values was quite broad and only in one case did the APE fall well below the desirable threshold (unlike the CV, which was always well above).
In total, 14 studies validated annulus counts in carp, thereby assessing their accuracy (Table 7). The scale, operculum, otolith and vertebra were the structures for which validation was achieved. This involved the follow-up of fish of known age, mark-recapture, marginal increment analysis, and the examination of length modes. The overall range of age classes for which validation was achieved was 1–7 for the scale (2–7 based on mark-recapture), 1–15 for the operculum (marginal increment analysis and length modes only), 1–15 for the otolith (1–12 and 14 as a combination of mark-recapture and fish of known age), and 1–7 for the vertebra (1–6 fish of known age). Except for the Czech Republic and Turkey, where carp is native and native/translocated, respectively, all other countries where validation studies were conducted fell into the species’ introduced range, where carp poses a medium or high risk level of impact on the aquatic ecosystem.
Discussion
According to the present review, scales have been both historically and traditionally the most widely-employed structure for ageing carp, even though in countries where the species carries a high risk of impact, use of alternative structures (i.e. operculum, and especially otolith, dorsal spine, vertebra and fin ray) has become increasingly common. The inconsistent evidence revealed by causal criteria analysis about the utility of scales and opercula to age carp successfully as opposed to otoliths, dorsal spines, vertebrae and (pectoral) fin rays emphasises the requirement for re-considering the routine use of ‘traditional/old-style’ ageing methods but also for ensuring validation of age estimates. This contention is further supported by the better precision achieved by otoliths, dorsal spines and fin rays compared to scales. For further studies on carp age and growth, it is therefore suggested that dorsal spines or pectoral fin rays should be selected as non-lethal ageing structures, and otoliths (and, possibly, vertebrae) when sacrificing the fish does not pose a logistic problem.
History
Given the century-long history of scientific studies on carp, it is not surprising that the entire range of available methods for ageing fish (i.e. scale, otolith, fin ray or spine, centrum or vertebral, and flat bone: Casselman 1983) has been used on this species. Yet, at the global level, the present findings indicate that the scale method has been predominant in age-growth studies on carp, regardless of the more recent advances in fish age determination with special emphasis on the use of the otolith (Campana and Thorrold 2001). There are several possible reasons for this somewhat ‘conservative’ status in the ageing of carp:
Anatomical features
The otolith method was originally developed and implemented for marine fishes and referred to also as the ‘sagitta’ method (Casselman 1983), because of the use of the sagittal pair of otoliths (Secor et al. 1991). In the case of carp, as previously discussed, it is the asteriscus pair of otoliths that is the most prominent and useful for annulus counting, but it is also more difficult to locate and extract relative to the sagittae of non-ostariophysans. This is evinced by the difficulties that have been encountered by early researchers (Table 2), leading in some cases to somewhat hasty conclusions as to the lack of usefulness of otoliths for ageing carp in general (Jones 1974; Hume et al. 1983). The unique structure of the ostariophysan vestibular apparatus in carp appears therefore to have been one of the factors contributing to the observed delay in the use of the otolith method in this species, both for annulus (asteriscus) and microincrement (lapillus) counting.
Limited longevity
As a result of using otoliths, mainly through their sectioning but often coupled with bomb radiocarbon dating (Campana 2001), several marine species (in many cases of high commercial fisheries value) have been shown to be much longer lived than previously thought (Campana 2001), with validated ages often in the range of 50–100 years (e.g. Black et al. 2005; Tracey et al. 2016). With regard to longevity, estimated ages of 35 (Bajer and Sorensen 2010) and 23 years (Brown et al. 2003) have been reported for carp based on otolith sections, although the oldest (mark-recapture validated) age using this method has been up to 14 years (Brown et al. 2004). On the other hand, published otolith-based age-growth data on carp have so far been limited to the 1–19 years old range (Vilizzi and Copp 2017), indicating the need for further research (especially regarding validation) into this ageing method. At the same time, references to exceptionally old carp, albeit occasionally encountered in the literature, have been so far mostly anecdotal and based on individuals reared in captivity (e.g. Flower 1935; Einsele 1956), hence in habitat conditions that are likely to differ profoundly from those of populations in the wild. Also, findings about carp longevity in some cold-climate water bodies of North America (Köppen-Geiger climate class D: Peel et al. 2007), albeit most likely not as pronounced as that of some marine species, are relatively recent (Bajer and Sorensen 2010) and may have therefore been responsible for the lack of adoption (as originally suggested by Vilizzi 1997) of the more advanced methods of otolith sectioning (possibly coupled with bomb radiocarbon dating) in this species so far.
Carp status
The status of carp as native, native/translocated or introduced has clearly affected the interest (or lack thereof) revolving around the use of ageing structures other than scales. Thus, in countries where carp is native, the scale method has been highly predominant, and this may have been an outcome of the more limited interest in carp as an invasive species. On the other hand, the stark predominance of studies relying upon alternative ageing structures to the scale (and operculum) in countries where carp is introduced is a reflection of the level of risk posed by this species on the aquatic ecosystem (Vilizzi et al. 2015).
Relative utility
In the present study, causal criteria analysis has provided for an objective, evidence-based comparative evaluation of the relative utility of ageing structures in carp. The inconsistent evidence reached for the scale and the operculum reflects the difficulties often encountered in annulus identification in these structures. This suggests that alternative structures to the scale should be used whenever carp is to be released after capture, and to the operculum when sacrificing the fish does not represent a problem. Regardless, the scale alone (Cazorla and Pizarro 2000; Colautti and Freyre 2001; Oyugi et al. 2011) and the scale and operculum together (Tempero et al. 2006) were recently used successfully in validated studies (Table 7). However, the populations of carp were in the former case ‘short-lived’ (1–7 years) and in the latter ‘medium-lived’ (1–12 years), and always in temperate climates (types Cfa and Cfb, Köppen-Geiger system) (note that the ‘relative longevity’ of these populations is gauged after Vilizzi and Copp 2017).
Support for the hypothesis of successful annulus identification/counting was provided for the (non-lethal) dorsal spine and fin ray methods and for the (lethal) otolith and vertebra methods. In a recent comparative study, pectoral fin rays and the dorsal spine were found to provide clear and easy-to-enumerate annuli, with the latter structure being preferred because of its more regular shape (Yates et al. 2016). These results corroborate previous findings on the reliability of both these non-lethal ageing structures (Phelps et al. 2007; Weber and Brown 2011), even though underestimation of age from the dorsal spine relative to the otolith has been reported (Colvin et al. 2012). With regard to the otolith and vertebra, the former was found to be more useful in a comparative study (Phelps et al. 2007), whereas in another study both structures were considered of similar utility (Yılmaz and Polat 2008). On the other hand, the majority of (recent) studies focusing on the use of otoliths alone have been able to age successfully carp with estimated (albeit not validated) ages over 20 years (Brown et al. 2003; Bajer and Sorensen 2010), even though there have been disagreements as to the value of using either whole or sectioned asterisci. In this respect, Brown et al. (2004) confidently and successfully aged carp using otolith sections, hence without facing the problems relating to the crowding of annuli at the edge (in older fish) as emphasised in other studies (Vilizzi and Walker 1999; Winker et al. 2010). Nor did Brown et al. (2004) report on the presence of ‘discontinuities’ (Vilizzi and Walker 1999; Winker et al. 2010), especially near the otolith core, which led Vilizzi and Walker (1999) to distinguish three morphological classes of asterisci. Recent findings of biannual growth zone formation based on the examination of the whole asteriscus (Winker et al. 2010), as opposed to complete unreadability reported for this structure (Yates et al. 2016), further obscure the picture and suggest that the use of whole and/or sectioned asterisci (or their lack of usefulness for ageing carp altogether) could be driven by population- or waterbody-specific factors.
Precision
A most notable outcome of the overall assessment of precision in carp ageing estimates was the failure of scales in all cases to provide levels of either APE or CV below desirable thresholds. Whereas in the case of the operculum, the target was reached but only in one study. These findings mirror the outcomes of causal criteria analysis (also considering that this included five of the eleven studies assessing precision: Tables 3 and 5), and the between-structure comparisons of the scale versus dorsal spine, scale versus fin ray, and operculum versus otolith further support the use of the dorsal spine or fin ray in lieu of the scale as non-lethal structures, and of the otolith versus the operculum as a lethal structure, as indicated above (note that no operculum versus vertebra comparison was available).
In the present study, selection of two threshold values for the CV and evaluation of precision in general reflects the adoption of a more flexible approach than one merely dictated by ‘cast-in-stone’ reference values. Thus, the recommended (higher) CV threshold of 7.6% was retained given its meta-analytical foundation, although slight preference was given to the (lower and more conservative) APE threshold of 5%, which has been suggested as a “reference point for many fishes of moderate longevity and reading complexity” (Campana 2001, p. 224). In this respect, carp can effectively be categorised as a fish of moderate longevity (Vilizzi and Copp 2017), especially when compared to marine species, and the reading complexity of its ageing structures also match the above definition. Further, it is argued that the very strict 5% threshold value for the APE expected in some ageing laboratories (Morison et al. 1998), whilst a solid indicator of the reproducibility of annulus counts, may by itself be biased by laboratory-specific protocols for annulus interpretation (hence, training of interpreters), which may differ from other laboratories (cf. Yates et al. 2016).
Except for two studies (Vilizzi et al. 1998; Yılmaz and Polat 2008), precision was overall satisfactory for the otolith and in several cases below reference thresholds. With the exception of one study (Yılmaz and Polat 2008), the target and close-to-target precision values achieved with the dorsal spine are a strong indicator of the reliability of this ageing structure. However, as the only available comparison between the dorsal spine and pectoral fin ray did not achieve target precision (Weber and Brown 2011), a final evaluation of the ‘best’ structure (if any) to choose between the two should be based on additional considerations related to morphology and preparation (see below: Directions for future research). Finally, the target value for precision reached in the only study dealing with the vertebra points to the potential value of this structure as an alternative to the otolith, although the lack of an evaluation of precision between these two structures again involves additional consideration as to which one would likely prove the most valuable.
Accuracy
Given the very large number of published studies on the age and growth of carp (Vilizzi and Copp 2017), it is remarkable that only a minor proportion of them has provided an evaluation of accuracy. Also, of the studies addressing accuracy, about three-quarters validated absolute age (i.e. through the follow-up of fish of known age, mark-recapture and, to some extent, assessment of discrete length modes), with the rest validating growth increment formation only (i.e. by means of MIA). Whilst validation of absolute ages may often represent a demanding and costly task (as in mark-recapture studies) or prove unfeasible (as with fish of known age), the use of MIA can be easily achieved under almost any circumstances (e.g. limited funding, lack of access to fisheries enterprises). It is therefore argued that MIA should represent the ‘minimal requirement’ for future age-growth studies on carp.
Excluding the two studies on carp reared in captivity and including only those achieving validation of absolute ages, the scale and the otolith were the structures for which the most rigorous assessment of accuracy has been achieved so far. On the other hand, lack of validation of absolute ages based on the dorsal spine, fin ray or vertebra methods prevents drawing any further conclusions as to the ultimate reliability of these structures, and points to the necessity of future structure-specific validation studies (see Yates et al. 2016).
Directions for future research
The outcomes of the present study corroborate the conclusions of a recent evaluation of ageing structures for carp that scales are overall unreliable and that the dorsal spine and, secondarily, the pectoral fin ray represent more valid alternatives (Yates et al. 2016). On the other hand, the conclusions reached in that study about the otolith differ from the majority of the other studies relying on this structure and also with the present review.
Below, some suggestions and guidelines are provided that are meant to assist researchers in future ageing studies on carp:
-
Unless validated, the use of the scale is discouraged in favour of the dorsal spine or (pectoral) fin ray whenever a non-lethal structure is required. Inexpensive and fast preparation techniques for the dorsal spine and fin ray are available (Koch and Quist 2007). However, due to its more regular shape, use of the dorsal spine may be preferred to that of fin rays (Yates et al. 2016). Sections taken at ≤ 25% of the total length of the dorsal spine have been shown to provide the most precise age estimates, with the section plane located at the 25% threshold being regarded as quite satisfactory (Watkins et al. 2015). This finding indicates that dorsal spine disarticulation (or total amputation) can be avoided, resulting in less stress (and long-term handicap) to the fish. Also, partial regeneration of the dorsal spine following amputation has been shown to occur, albeit slowly and at a higher sectioning plane that the 25% distance from the base (Kalish-Achrai et al. 2017). Similar to the scale, age estimation based on the operculum is discouraged in favour of the otolith whenever sacrificing the fish does not pose a problem. Finally, the vertebra could be also used in lieu of the operculum, but more research is needed as to the reliability of this structure for ageing carp, including a more precise definition of which vertebra(e) are most suitable for annulus counting.
-
As a corollary to the above conclusions, it is argued that use of the dorsal spine (or fin ray) should not yet be considered a replacement for the otolith. This is contrary to the argument put forward by Watkins et al. (2015, p. 694) that “[s]upport for otoliths as the premier age estimation structure has waned as more efficient, nonlethal structures are verified”. In this respect, whilst estimated ages of up to 27 and 24 years have been provided by use of the dorsal spine and pectoral fin ray, respectively (Weber and Brown 2011; see also Jackson et al. 2007), estimated ages up to 35 years have been found with the otolith section method (Bajer and Sorensen 2010). Clearly, the current paucity of studies on potentially long-lived populations (especially those of the colder regions of North America) prevents drawing any further conclusions in regard. It is also suggested that the more advanced method of bomb radiocarbon dating should be trialled on carp otoliths (Campana 2001), even though it is unlikely that even longer-lived carp populations would achieve longevities similar to those of some marine fishes. Use of both the dorsal spine (or pectoral fin ray) and otolith (preferably sectioned, in case of long-lived populations) should therefore be attempted whenever populations of carp are investigated for the first time (e.g. Yates et al. 2016). In case of similarity in age estimates, use of the dorsal spine or pectoral fin ray may be preferable for practical reasons (Koch and Quist 2007), even when release of carp back into the waters is discouraged (e.g. http://www.dpi.nsw.gov.au/fishing/pests-diseases/freshwater-pests/species/carp/groups/recreational-fishers; accessed 30/12/2017). Finally, the potential relationship between the microstructure of the vateritic asterisci (Li et al. 2009) and the difficulties oftentimes encountered in successful annulus identification (as opposed to the annuli of the aragonitic sagittae in non-ostaryophysans) deserves investigation.
-
As highlighted in a recent review of carp growth at the global scale (Vilizzi and Copp 2017), the disparate ageing methods employed with over-reliance on the use of scales and ‘near-chronic’ lack of an evaluation of precision and accuracy may well represent a confounding effect for the refinement of population dynamics models based on age distributions and dynamic rate functions (Ricker 1975). This limitation could only be resolved through consistently-designed (albeit resource-demanding), global studies on carp age and growth and through the set-up of inter-laboratory programmes involving the creation of reference collections and quality control monitoring (Campana 2001). As recommended above, minimal validation of estimated ages (cf. MIA) should always be ensured, so as to avoid the shortcomings associated with ‘word-of-mouth’ scientific practice. In this respect, the adoption of appropriate ageing protocols for carp should be extended to all of its areas of distribution worldwide (hence, outside North America, Australia and South Africa, where ‘modern’ ageing methods have become routine). These include not only regions with warm and/or arid climates (e.g. Central and South America, Africa), where carp is predicted to expand further its invasive range of distribution (Zambrano et al. 2006; Crichigno et al. 2016; Maiztegui et al. 2016), but also those areas where the species plays an important commercial role for fisheries, as in Anatolia, Turkey (Gaygusuz et al. 2015) and in the Caspian Sea region (Abdullaev 2011; Amouei et al. 2013; Sedaghat et al. 2013).
References
Abdullaev A (2011) Number, age structure and growth of roach Rutilus caspicus (Yakovlev, 1870) and common carp Cyprinus carpio (Linnaeus, 1758) in the Divichi Firth (Agzybir Lake). Mosc State Univ Bull 4:15–20 (in Russian with English abstract)
Akyurt İ (1987) Studies on the population of mirror carp of the Kazan Lake. Ç Ü Ziraat Fakültesi Derg 3:323–340 (in Turkish with English abstract)
Almeida D, Ribeiro F, Leunda PA, Vilizzi L, Copp GH (2013) Effectiveness of FISK, an invasiveness screening tool for non-native freshwater fishes, to perform risk identification assessments in the Iberian Peninsula. Risk Anal 33:1404–1413
Amouei F, Valinassab T, Haitov A (2013) Age determination and morphological study using otoliths in Cyprinus carpio Linnaeus, 1758 in the Southern Caspian Sea. Iran J Fish Sci 12:749–758
Aydın R, Pala M, Yüksel F, Şen D (2009) Age determination of mirror carp (Cyprinus carpio L., 1758) on otoliths with broken and burnt method. J Fish Sci 3:51–57 (in Turkish with English abstract)
Bajer PG, Sorensen PW (2010) Recruitment and abundance of an invasive fish, the common carp, is driven by its propensity to invade and reproduce in basins that experience winter-time hypoxia in interconnected lakes. Biol Inv 12:1101–1112
Bajer PG, Sullivan G, Sorensen PW (2009) Effects of a rapidly increasing population of common carp on vegetative cover and waterfowl in a recently restored Midwestern shallow lake. Hydrobiol 632:235–245
Balon EK (1957) Age and growth of spawning school of the Danubian wild carp. Pol’nohospodártsvo (Bratislava) 4:961–986 (in Slovak with English abstract)
Balon EK (1995) The common carp, Cyprinus carpio: its wild origin, domestication in aquaculture, and selection as colour nishikigoi. Guelph Ichthyol Rev 3:1–55
Balon EK (1999) Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses). Environ Biol Fish 56:17–38
Bănărescu P (1964) Fauna of the Republic of Romania. Pisces—Osteichthyes, vol 13. Editura Academiei Republicii Populare Romîne, Bucuresti, pp 472–486 (in Romanian)
Beamish RJ (1981) Use of fin-ray sections to age walleye pollock, Pacific cod, and albacore, and the importance of this method. Trans Am Fish Soc 110:287–299
Beamish RJ, McFarlane GA (1983) The forgotten requirement for age validation in fisheries biology. Trans Am Fish Soc 112:735–743
Beddington JR, Kirkwood GP (2005) The estimation of potential yield and stock status using life-history parameters. Phil Trans R Soc B 360:163–170
Bhandari BS, Johal MS, Tandon KK (1993) Age and growth of Cyprinus carpio var. communis Linnaeus from Gobindsagar, Himachal Pradesh, India. Res Bull Panj Univ 43:151–167
Bishai HM, Labib WD (1978) Age and growth of mirror carp (Cyprinus carpio L.) at Serow Fish Farm. Bull Inst Oceanogr Fish 8:397–418
Black BA, Boehlert GW, Yoklavich MM (2005) Using tree-ring crossdating techniques to validate annual growth increments in long-lived fishes. Can J Fish Aquat Sci 62:2277–2284
Blair JM (2008) An investigation of koi carp (Cyprinus carpio) movement in the Waikato region using laser ablation otolith microchemistry. PhD Dissertation, University of Waikato
Blair JM, Hicks BJ (2012) Otolith microchemistry of koi carp in the Waikato region, New Zealand: a tool for identifying recruitment locations? Inland Waters 2:109–118
Bostanci D (2009) Otolith biometry-body length relationships in four fish species (chub, pikeperch, crucian carp, and common carp). J Freshw Ecol 24:619–624
Boyko SG (1950) Age determination in fishes based on examination of fin ray section. Progress Fish Cult 12:47–48
Britton JR, Boar RR, Grey J, Foster J, Lugonzo J, Harper DM (2007) From introduction to fishery dominance: the initial impacts of the invasive carp Cyprinus carpio in Lake Naivasha, Kenya, 1999 to 2006. J Fish Biol 71:239–257
Brown P, Walker TI (2004) CARPSIM: stochastic simulation modelling of wild carp (Cyprinus carpio L.) population dynamics, with applications to pest control. Ecol Model 176:83–97
Brown P, Sivakumaran KP, Stoessel D, Giles A, Green C, Walker T (2003) Carp population biology in Victoria. Report 56, February 2003. Marine and Freshwater Resources Institute, Department of Primary Industries, Snobs Creek, Victoria, Australia http://www.feral.org.au/wp-content/uploads/2012/01/Brown2003_CarpPopBiology.pdf. Accessed 30 Dec 2017
Brown P, Green C, Sivakumaran KP, Stoessel D, Giles A (2004) Validating otolith annuli for annual age determination of common carp. Trans Am Fish Soc 133:190–196
Brown P, Sivakumaran KP, Stoessel D, Giles A (2005) Population biology of carp (Cyprinus carpio L.) in the mid-Murray River and Barmah Forest Wetlands. Australia. Mar Freshw Res 56:1151–1164
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol 59:197–242
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci 58:30–38
Carlton WG, Jackson WB (1964) The use of spines for age determination of fish. Turtox News 42:282–283
Carlton WG, Jackson WB (1968) The eye lens as an age indicator in carp. Copeia 3:633–666
Casal CMV (2006) Global documentation of fish introductions: the growing crisis and recommendations for action. Biol Inv 8:3–11
Casas L, Szűcs R, Vij S, Goh CH, Kathiresan P, Németh S, Bercsényi M, Orbán L (2013) Disappearing scales in carps: re-visiting Kirpichnikov’s model on the genetics of scale pattern formation. PLoS ONE 8:e83327
Casselman JM (1983) Age and growth assesment of fish from their calcified structures—techniques and tools. National Oceanic and Atmospheric Administration Technical Report NMFS 8, pp 1–17
Cazorla AL, Pizarro G (2000) Age and growth of the common carp Cyprinus carpio (L.) in the irrigation system of the Colorado River Valley, Buenos Aires Province, Argentina. Nat Neotropic 31:61–71
Çetinkaya O (1992) Studies on the carp population (Cyprinus carpio L., 1758) in Akşehir Lake I. Growth, length-weight relationship and condition. Doğa Türk Zool Derg 16:13–29 (in Turkish with English abstract)
Çetinkaya O, Elp M, Güzel S (1995–1999) Structure and growth properties of mirror carp (Cyprinus carpio L., 1758) populations introduced to water resources in Lake Van Basin, Turkey. Istanbul Univ J Aquat Prod (Special issue) 9–13 (1–10):123–138 (in Turkish with English abstract)
Çetinkaya O, Sarı M, Arabacı M, Şen F, Duyar HA (1995b) Studies on fish populations of the Karasu River, Lake Van Basin, Turkey. Yüzüncü Yıl Üniv Ziraat Fak Derg 5:189–202 (in Turkish with English abstract)
Chistiakov DA, Voronova NV (2009) Genetic evolution and diversity of common carp Cyprinus carpio L. Central Europe. J Biol 4:304–312
Christenson LM, Smith LL (1965) Characteristics of fish populations in upper Mississippi River backwater areas. US Fish and Wildlife Service Circular, 212, Washington
Cochrane KL (1985) The population dynamics and sustainable yield of the major fish species in Hartbeespoort Dam. PhD Dissertation, University of the Witwatersrand
Çolakoğlu S, Akyurt I (2011) Population structure and growth properties of mirror carp (Cyprinus carpio L., 1758) in Bayramiç Dam Lake. Istanbul Univ J Fish Aquat Sci 26:27–46 (in Turkish with English abstract)
Colautti D, Freyre LR (2001) Growth of carp (Cyprinus carpio) in Laguna de Lobos, Buenos Aires, Argentina. Revista de Ictiologia 9:5–11 (in Spanish with English abstract)
Colvin ME, Pierce CL, Beck L (2012) Age structure and growth of invasive common carp populations in the Malheur National Wildlife Refuge. Report to US Fish and Wildlife Service Malheur National Wildlife Refuge, USA http://mec685.cfr.msstate.edu/pdfs/Malheur_Carp_Age_Growth_Report.pdf. Accessed 30 Dec 2017
Copp GH, Bianco PG, Bogutskaya NG, Erős T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kováč V, Moreno-Amich R, Naseka AM, Peňáz M, Povž M, Przybylski M, Robillard M, Russell IC, Stakėnas S, Šumer S, Vila-Gispert A, Wiesner C (2005) To be, or not to be, a non-native freshwater fish? J Appl Ichthyol 21:242–262
Coulter DP, Jolley JC, Edwards KR, Willis DW (2008) Common carp (Cyprinus carpio) population characteristics and recruitment in two Nebraska sandhill lakes. Trans Nebraska Acad Sci 31:35–41
Crichigno S, Cordero P, Blasetti G, Cussac V (2016) Dispersion of the invasive common carp Cyprinus carpio in southern South America: changes and expectations, westward and southward. J Fish Biol 89:403–416
Crivelli A (1980) The eye lens weight and age in the common carp, Cyprinus carpio L. J Fish Biol 16:469–473
Crook DA, Gillanders BM (2006) Use of otolith chemical signatures to estimate carp recruitment sources in the mid-Murray River, Australia. River Res Appl 22:871–879
Crook DA, Macdonald JI, McNeil DG, Gilligan DM, Asmus M, Maas R, Woodhead J (2013) Recruitment sources and dispersal of an invasive fish in a large river system as revealed by otolith chemistry analysis. Can J Fish Aquat Sci 70:953–963
Das SM, Fotedar J (1965) Studies on the scales, age and growth of freshwater fishes of Kashmir. Part I: Cyprinus carpio specularis Linn. Ichthyologica 4:79–91
Deng Z, Yu Z, Xu Y, Wei X, Zhao Y (1981) On the age and growth of the main commercial fishes collected from Hansui River. Trans Chin Ichthyol Soc 1:97–116 (in Chinese with English abstract)
Diggle J, Day J, Bax N (2004) Eradicating European carp from Tasmania and implications for national European carp eradication. Hobart: Inland Fisheries Service www.ifs.tas.gov.au/about-us/publications/eradicating-european-carp-from-tasmania-and-implications-for-national-european-carp-eradication. Accessed 30 Dec 2017
Effendie MI (1968) Growth and food habits of carp, Cyprinus carpio L., in Clear Lake, Iowa. MSc Dissertation, Iowa State University
Einsele W (1956) On the longevity of our fishes. Oesterreichische Fisch 9:25–31 (in German)
El-Fiky NK (1993) Development of the scales in Cyprinus carpio. J Egypt Ger Soc Zool 10(B):137–149
English TS (1952) Method of sectioning carp spines for growth studies. Prog Fish Cult 14:36
Fernández-Delgado C (1990) Life history patterns of the common carp, Cyprinus carpio, in the estuary of the Guadalquivir River in south-west Spain. Hydrobiol 206:19–28
Flower SS (1935) Further notes on duration of life in animals. I. Fishes: as determined by otolith and scale-readings and direct observation on living individuals. Proc Zool Soc Lond 1935:265–304
Frey DG (1942) Studies on Wisconsin Carp, 1. Influence of age, size, and sex on time of annulus formation by 1936 year class. Copeia 1942:214–223
Gaygusuz Ö, Tarkan AS, Aydın H, Dorak Z, Top N, Karakuş U, Vilizzi L (2015) Stocking of common carp (Cyprinus carpio) into newly-established reservoirs may create new introduction pathways for non-native fish. Turk J Fish Aquat Sci 15:833–840
Gilligan DM, Schiller C (2003) Downstream transport of larval and juvenile fish in the Murray River. NSW Fisheries Final Report Series No. 50, NSW Fisheries Office of Conservation, Australia www.dpi.nsw.gov.au/__data/assets/pdf_file/0009/545634/FFRS-50_Gilligan-and-Schiller-2003.pdf. Accessed 30 Dec 2017
Gümüş A (1998) Age validation in hard structures of mirror carp (Cyprinus carpio L.) by increment analysis method. PhD Dissertation (in Turkish with English abstract)
Hilborn R, Walters CJ (eds) (2013) Quantitative fisheries stock assessment: choice, dynamics and uncertainty. Springer, New York
Hoffbauer C (1898) Age determination of carp with its scales. Allg Fisch Ztg 23:341–343 (in German)
Hoffbauer C (1905) Further contributions to the age and growth determination of the fish, especially of the carp. Zeit Fisch 3:111–142 (in German)
Hume DJ, Fletcher AR, Morison AK (1983) Carp program-final report. Report No. 10. Arthur Rylah Institute for Environmental Research, Fisheries and Wildlife Division, Ministry for Conservation, Victoria, Australia
Huntingford FA, Borcato FL, Mesquita FO (2013) Identifying individual common carp Cyprinus carpio using scale pattern. J Fish Biol 83:1453–1458
Hutchison M, McLennan M, Chilcott K, Norris A and Stewart D (2012). Validating the age of carp from the northern Murray-Darling Basin. PestSmart Toolkit publication, Invasive Animals Cooperative Research Centre, Canberra, Australia
Izzo C, Bertozzi T, Gillanders BM, Donnellan SC (2014) Variation in telomere length of the common carp, Cyprinus carpio (Cyprinidae), in relation to body length. Copeia 2014:87–94
Jackson SW Jr (1955) Rotenone survey of black hollow on lower Spavinaw Lake, November 1953. Proc Oklahoma Acad Sci 35:10–14
Jackson ZJ, Quist MC, Larscheid JG, Thelen EC, Hawkins MJ (2007) Precision of scales and dorsal spines for estimating age of common carp. J Freshw Ecol 22:231–239
Jearld A Jr (1983) Age determination. In: Johnson DL, Lampton SS (eds) Fisheries techniques. American Fisheries Society, Bethesda, pp 301–324
Jester DB (1974) Life history, ecology, and management of the carp, Cyprinus carpio Linnaeus, in Elephant Butte Lake. New Mexico State University, Agricultural Experiment Station, Research Report 273
Jestin JM, Lefrançois O, Renoncourt L (1985) Influence of the impoundment of dams on the individual growth of carp (Cyprinus carpio) and bream (Abramis brama). Verh Int Ver Theor Angew Limnol 22:2598–2604 (in French)
Johal MS, Novak J, Oliva O (1984) Notes on the growth of the common carp (Cyprinus carpio) in Northern India and in Central Europe. Věst Česk Společ Zool 48:24–38
Johal MS, Rawal YK, Kaur A, Kaur A (2014) Ultrastructure of the focus region of the regenerated cycloid scale of an exotic fish, Cyprinus carpio communis L. as a possible key to comprehensive understanding of populations. Curr Sci 106:744–748
Jones W (1974). Age determination and growth studies of four species of fish from the River Murray. BSc Dissertation, University of Adelaide
Kalish-Achrai N, Monsonego-Ornan E, Shahar R (2017) Structure, composition, mechanics and growth of spines of the dorsal fin of blue tilapia Oreochromis aureus and common carp Cyprinus carpio. J Fish Biol 90:2073–2096
Karakoç R, Sarıhan E (1987) A study on growth performances and fishing composition of pike-perch (Stizostedion lucioperca (L.), 1758) and mirror carp (Cyprinus carpio (L.), 1758) populations in Seyhan Dam Lake. Çukurova Üniv Fen Mühen Bilim Derg 1:69–80 (in Turkish with English abstract)
Katzenmeyer ED (2010) Fish growth responses to a changing environment: effects of aquatic nuisance species and environmental conditons in a shallow, eutrophic lake. Iowa State University, Graduate Theses and Dissertations, Paper 11827, Iowa, USA http://lib.dr.iastate.edu/etd/11827. Accessed 30 Dec 2017
Khan MA, Khan S (2009) Comparison of age estimates from scale, opercular bone, otolith, vertebrae and dorsal fin ray in Labeo rohita (Hamilton), Catla catla (Hamilton) and Channa marulius (Hamilton). Fish Res 100:255–259
Kırankaya ŞG (2007) A comparative study on growth, reproduction and feeding biology of mirror carp, wild carp (Cyprinus carpio, L., 1758) and Prussian carp [Carassius gibelio (Bloch, 1782)] in Gelingüllü Dam Lake (Yozgat-Turkey). PhD Dissertation, Hacettepe University (in Turkish with English abstract)
Kırankaya ŞG, Ekmekçi FG (2004) Growth properties of mirror carp (Cyprinus carpio L., 1758) introduced into Gelingüllü Dam Lake. Turk J Vet Anim Sci 28:1057–1064 (in Turkish with English abstract)
Kirpitchnikov VS (1999) Genetics and breeding of common carp. INRA, Paris
Koch JD, Quist MC (2007) A technique for preparing fin rays and spines for age and growth analysis. N Am J Fish Manage 27:782–784
Koehn JD, Todd CR, Zampatti BP, Stuart IG, Conallin A, Thwaites L, Ye Q (2017) Using a population model to inform the management of river flows and invasive carp (Cyprinus carpio). Env Manage. https://doi.org/10.1007/s00267-017-0855-y
Krumholz LA (1956) Observations on the fish population of a lake contaminated by radioactive wastes. Bull Am Mus Nat Hist 110:277–368
Lechelt JD, Bajer PG (2016) Modeling the potential for managing invasive common carp in temperate lakes by targeting their winter aggregations. Biol Inv 18:831–839
Li Z, Gao Y, Feng Q (2009) Hierarchical structure of the otolith of adult wild carp. Mater Sci Eng C 29:919–924
Li S, Gao Y, Luo J, Cao Y, Yang L, Zhang X, Yan L, Du F (2011) The thermoluminescence of carp otoliths: A fingerprint in identification of lake pollution. Afr J Biotechnol 10:18440–18449
Liang ZC, Yang SW, Wu LQ (1993) Studies on the growth characteristics of common carp (Cyprinus carpio) and crucian carp (Carassius auratus) in Biliuhe Reservoir and its utilization in fishery. J Dalian Fish Univ 8:33–42 (in Chinese with English abstract)
Linfield RSJ (1982) Studies on the growth of common carp, Cyprinus carpio L., in a Lake fishery. Fish Manage 13:45–64
Lubinski KS, Jackson SD, Hartsfield BN (1984) Age structure and analysis of carp populations in the Mississippi and Illinois Rivers. Illinois Natural History Survey, Aquatic Biology Technical Report 1984(9)
Macdonald JI, Crook DA (2014) Nursery sources and cohort strength of young-of-the-year common carp (Cyprinus carpio) under differing flow regimes in a regulated floodplain river. Ecol Freshw Fish 23:269–282
Macdonald J, Crook D, McNeil DG (2010) Identification of carp recruitment hotspots in the LAchlan River using otolith chemistry. Report to the Invasive Animals CRC and Lachlan CMA, prepared by SARDI Aquatic Sciences. Invasive Animals Cooperative Research Centre, Canberra. SARDI Publication No. F2009/000682-1. SARDI Research Report Series No. 434 www.researchgate.net/publication/247768728_Identification_of_Carp_Recruitment_Hotspots_in_the_Lachlan_River_Using_Otolith_Chemistry. Accessed 30 Dec 2017
Macdonald JI, McNeil DG, Crook DA (2012) Asteriscus v. lapillus: comparing the chemistry of two otolith types and their ability to delineate riverine populations of common carp Cyprinus carpio. J Fish Biol 81:1715–1729
Maiztegui T, Baigún CRM, Garcia de Souza JR, Minotti P, Colautti DC (2016) Invasion status of the common carp Cyprinus carpio in inland waters of Argentina. J Fish Biol 89:417–430
Mann RHK (1991) Growth and production. In: Winfield J, Nelson JS (eds) Cyprinid fishes: Systematics, biology and exploitation. Chapman and Hall, London, pp 456–482
Marlborough D (1967) Some studies on common carp (Cyprinus carpio L.) and crucian carp (Carassius carassius L.) in a small Middlesex pond. Lon. Nat. 46:76–81
Marr SM, Ellender BR, Woodford DJ, Alexander ME, Wasserman RJ, Ivey P, Zengeya T, Weyl OL (2017) Evaluating invasion risk for freshwater fishes in South Africa. Bothalia Afr Biodivers Cons 47:a2177
Matsui I (1949) Studies on the scales of the important freshwater fishes in Manchuria. J Shimonoseki Coll Fish 11:33–49 (in Japanese with English abstract)
McConnell WJ (1952) The opercular bone as an indicator of age and growth of the carp, Cyprinus carpio Linnaeus. Trans Am Fish Soc 81:138–149
Meunier FJ, Pascal M (1981/1982) Experimental study of cyclical growth in fin rays of carp (Cyprinus carpio L.). Preliminary results. Aquaculture 26:23–40 (in French with English abstract)
Morison AK, Robertson SG, Smith DG (1998) An integrated system for production fish aging: image analysis and quality assurance. N Am J Fish Manage 18:587–598
Nichols S, Webb A, Norris R, Stewardson M. (2011) Eco Evidence analysis methods manual: A systematic approach to evaluate causality in environmental science eWater Cooperative Research Centre, Canberra https://toolkit.ewater.org.au/Tools/DownloadDocumentation.aspx?id=1000301. Accessed 30 Dec 2017
Norris RH, Webb JA, Nichols SJ, Stewardson NJ, Harrison ET (2012) Analyzing cause and effect in environmental assessments: using weighted evidence from the literature. Freshwat Sci 31:5–21
Okumuş İ, Tekelioğlu N (1987) A study on the growth properties of Sera Lake carp]. Çukurova Üniv Ziraat Fak Derg 3:1–14 (in Turkish with English abstract)
Oliva O (1955) Contribution to the biology and growth of the carp in back-waters of the River Elbe Region. Universitas Carolina, Biologica 1:225–273 (in Czech with English abstract)
Omar AM, Amohamed SK (2016) Comparative morphological studies on the otoliths (ear stones or crystals) in some marine and fresh water fishes. Int J Fish Aquat Stud 4:512–517
Oyugi DO, Cucherousset J, Ntiba MJ, Kisia SM, Harper DM, Britton JR (2011) Life history traits of an equatorial common carp Cyprinus carpio population in relation to thermal influences on invasive populations. Fish Res 110:92–97
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Sys Sci 11:1633–1644
Perdikaris C, Koutsikos N, Vardakas L, Kommatas D, Simonović P, Paschos I, Detsis V, Vilizzi L, Copp GH (2016) Risk screening of non-native, translocated and traded aquarium freshwater fishes in Greece using Fish Invasiveness Screening Kit. Fish Manage Ecol 23:32–43
Phelps QE (2006) Population dynamics of common carp in eastern South Dakota glacial lakes. MSc Dissertation, South Dakota State University
Phelps QE, Edwards KR, Willis DW (2007) Precision of five structures for estimating age of common carp. N Am J Fish Manage 27:103–105
Phelps QE, Graeb BDS, Willis DW (2008) First year growth and survival of common carp in two glacial lakes. Fish Manage Ecol 15:85–91
Pinilla GA, Vargas P, Patiño E (1992) Population features of the carp (Cyprinus carpio L.) in the Laguna Fuquene. Bol Eutropica 25:28–41 (in Spanish with English abstract)
Prochelle O, Campos H (1985) The biology of the introduced carp Cyprinus carpio L., in the River Caymapu, Valdivia, Chile. Stud Neotrop Fauna Env 20:65–82
Raina HS (1987) A biological note on the introduced common carp in the temperate waters of Kashmir. Indian J Fish 34:114–119
Rehder DD (1959) Some aspects of the life history of the carp, Cyprinus carpio, in the Des Moines River, Boone County, Iowa. Iowa State J Sci 34:11–26
Ricker WL (1975) Computation and interpretation of biological statistics of fish populations. Fish Res Board Can Bull 19:1–382
Sanchez C Jr. (1970) Life history and ecology of carp, Cyprinus carpio Linnaeus in Elephant Butte Lake, New Mexico. MSc Dissertation, New Mexico State University
Sandoz O’R (1960) A pre-impoundment study of Arbuckle Reservoir Rock Creek Murray County Oklahoma. Oklahoma Fisheries Research Laboratory, Oklahoma, USA, Report Number 77
Sarıhan E (1980) A study on the development of the smallest catch size of mirror carp reared in Seyhan Dam Lake. Çağdaş Tarim Tekn Derg 9–10–11:24–28 (in Turkish with English abstract)
Schoonover R, Thompson WH (1954) A post-impoundment study of the fisheries resources of Fall River Reservoir, Kansas. Trans Kansas Acad Sci 57:172–179
Secor DH, Dean JM, Laban EH (1991) Manual for otolith removal and preparation for microstructural examination. The Belle W. Baruch Institute for Biology and Coastal Research, Columbia
Sedaghat S, Hoseini SA, Larijani M, Ranjbar KS (2013) Age and growth of common carp (Cyprinus carpio Linnaeus, 1758) in Southern Caspian Sea, Iran. World J Fish Mar Sci 5:71–73
Şen F (2001) A study on the carp (Cyprinus carpio L., 1758) population on the Lake Nazik (Ahlat-Bitlis-Turkey). PhD Dissertation, Atatürk University (in Turkish with English abstract)
Smith BB, Walker KF (2003) Validation of the ageing of 0 + carp (Cyprinus carpio L.). Mar Freshw Res 54:1005–1008
Starrett WC, Fritz AW (1965) A biological investigation of the fishes of Lake Chautauqua, Illinois. Illinois Nat Hist Surv Bull 29:1–104
Talaat KMM, Oláh J (1986) Fishery studies on Cyprinus carpio L. in Hungarian inland waters 1. Reliability of age determination using the scales of Cyprinus carpio L. Aquacult Hung (Szarvas) 5:235–240
Temizer IA, Şen D (2008) Compared age determination on bony structures of mirror carp (Cyprinus carpio L., 1758) inhabiting Keban Dam Lake. Sci Eng J Fırat Univ 20:57–66 (in Turkish with English abstract)
Tempero GW, Ling N, Hicks BJ, Osborne MW (2006) Age composition, growth and reproduction of koi carp (Cyprinus carpio) in the lower Waikato region, New Zealand. NZ J Mar Freshw Res 40:571–583
Tracey DM, Andrews AH, Horn PL, Neil HL (2016) Another New Zealand centenarian: age validation of black cardinalfish (Epigonus telescopus) using lead–radium and bomb radiocarbon dating. Mar Freshw Res 68:352–360
Tsimenide N (1978) Preliminary report on age and growth of the carp in Vistonis Lake, Greece. Thalassographica 1:53–63 (in Greek with English abstract)
van Leeuwenhoek A (1798) On the nature of the scales of fishes, and how the age of those animals may be determined by observation of the scales; the author’s reasonings and opinion respecting the longevity of this part of the animal creation. In: van Leeuwenhoek A. The select work of Antony van Leeuwenhoek, containing his microscopical discoveries in many of the works of nature. Translated from the Dutch and Latin editions published by the author by Samuel Hoole. Henry Fry, London, pp 89–96
Van Oosten J (1928) Life history of the lake herring (Leucichthys artedi Lesueuer) of Lake Huron as revealed from its scales with a critique of the scales method. Bull US Bureau Fish 44:265–428
Vilizzi L (1997) Age, growth and early life history of carp (Cyprinus carpio L.) in the River Murray, South Australia. PhD Dissertation, University of Adelaide
Vilizzi L (1998) Age, growth and cohort composition of 0 + carp in the River Murray, Australia. J Fish Biol 52:997–1013
Vilizzi L (2012) The common carp, Cyprinus carpio, in the Mediterranean Region: Origin, distribution, economic benefits, impacts and management. Fish Manage Ecol 19:93–110
Vilizzi L, Copp GH (2017) Global patterns and clines in the growth of common carp Cyprinus carpio. J Fish Biol. 91:3–40
Vilizzi L, Tarkan AS (2015) Experimental evidence for the effects of common carp (Cyprinus carpio L., 1758) on freshwater ecosystems: a narrative review with management directions for Turkish inland waters. J Limnol Freshw Fish Res 1:123–149
Vilizzi L, Walker KF (1995) Otoliths as potential indicators of age and growth in carp, Cyprinus carpio L. (Cyprinidae: Teleostei). Trans R Soc S Aust 115:97–98
Vilizzi L, Walker KF (1998) Age profile of carp (Cyprinus carpio L.) in Lake Crescent, Tasmania. Papers Proc R Soc Tasman 132:1–8
Vilizzi L, Walker KF (1999) Age and growth of the common carp, Cyprinus carpio L. (Cyprinidae), in the River Murray, Australia: validation, consistency of age interpretation and growth models. Environ Biol Fish 54:77–106
Vilizzi L, Walker KF, Jain T, McGlennon D, Tsymbal V (1998) Interpretability and precision of annulus counts for calcified structures in carp, Cyprinus carpio L. Arch Hydrobiol 143:121–127
Vilizzi L, Tarkan AS, Copp GH (2015) Experimental evidence from causal criteria analysis for the effects of common carp Cyprinus carpio on freshwater ecosystems: a global perspective. Rev Fish Sci Aquac 23:253–290
Vostradovský IJ (1962) The growth of carp (Cyprinus carpio) in Lipno dam reservoir (1958–1959) and some opinion from its exploitation. Práce VÚR Vodňany 1:135–156 (in Czech with English abstract)
Wang S (1983) On the age and growth of the carp (Cyprinus carpio L.) in Hurleg Lake of Qinghai Province. Acta Zool Sin 29:59–65 (in Chinese with English abstract)
Watkins CJ, Klein ZB, Terrazas MM, Quist MC (2015) Influence of sectioning location on age estimates from common carp dorsal spines. NAJ Fish Manage 35:690–697
Webb AJ, Miller KA, King EL, Little SC, Stewardson MJ, Zimmerman JK, LeRoy PN (2013) Squeezing the most out of existing literature: a systematic re-analysis of published evidence on ecological responses to altered flows. Freshw Biol 58:2439–2451
Weber MJ, Brown ML (2011) Comparison of common carp (Cyprinus carpio) age estimates derived from dorsal fin spines and pectoral fin rays. J Freshw Ecol 26:195–202
Weber MJ, Brown ML, Willis DW (2010) Spatial variability of common carp populations in relation to lake morphology and physicochemical parameters in the upper Midwest United States. Ecol Freshw Fish 19:555–565
Weber MJ, Hennen MJ, Brown ML (2011) Simulated population responses of common carp to commercial exploitation. NAJ Fish Manage 31:269–279
Weber MJ, Brown ML, Wahl DH, Shoup DE (2015) Metabolic theory explains latitudinal variation in common carp populations and predicts responses to climate change. Ecosphere 6:1–16
Weber MJ, Hennen MJ, Brown ML, Lucchesi DO, Sauver TRS (2016) Compensatory response of invasive common carp Cyprinus carpio to harvest. Fish Res 179:168–178
Welcomme RL (1988) International introductions of inland aquatic species. FAO Fisheries Technical Paper 294
Wichers WF (1976) Population dynamics and commercial potential of commercial fish species in impoundments. Wyoming Game and Fish Department, Cheyenne, USA
Winker H, Weyl OLF, Booth AJ, Ellender BR (2010) Validating and corroborating the deposition of two annual growth zones in asteriscus otoliths of common carp Cyprinus carpio from South Africa’s largest impoundment. J Fish Biol 77:2210–2228
Winker H, Weyl OLF, Booth AJ, Ellender BR (2011) Life history and population dynamics of invasive common carp, Cyprinus carpio, in a large turbid African impoundment. Mar Freshw Res 62:1270–1280
Yates JR, Watkins CJ, Quist MC (2016) Evaluation of hard structures used to estimate age of common carp. Northwest Sci 90:195–205
Yılmaz S, Polat N (2008) Evaluation of different bony structures for age determination of common carp Cyprinus carpio L., 1758. Süleyman Demirel Üniv Fen Edebiyat Fak Fen Derg (E-Dergi) 3:149–161 (in Turkish with English abstract)
Yılmaz S, Yazıcıoğlu O, Polat N (2012) Age and growth properties of common carp (Cyprinus carpio L., 1758) from Bafra Balık Lakes (Samsun, Turkey). The Black Sea J Sci 2:1–12 (in Turkish with English abstract)
Yüce S, Gündüz F, Demirol F, Çelik B, Alpaslan K, Çoban MZ, Aydın R, Şen D (2016) Some population parameters of mirror carp (Cyprinus carpio L., 1758) inhabiting Atatürk Dam Lake. LIMNOFISH–J Limnol Freshw Fish Res 2:31–41 (in Turkish with English abstract)
Zambrano L, Martinez-Meyer E, Menezes N, Townsend Peterson A (2006) Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Can J Fish Aquat Sci 63:1903–1910
Acknowledgements
I wish to thank G.H. Copp (Cefas, Lowestoft, UK) for providing valuable feedback on a final draft of this study, and M. Przybylski (University of Łódź) for assistance with some references in Russian.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vilizzi, L. Age determination in common carp Cyprinus carpio: history, relative utility of ageing structures, precision and accuracy. Rev Fish Biol Fisheries 28, 461–484 (2018). https://doi.org/10.1007/s11160-018-9514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-018-9514-5