Abstract
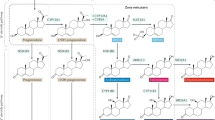
The biosynthesis of steroid hormones requires the coordinated expression of the enzymes that comprise the pathways via which specific hormones are synthesized. These pathways and their associated enzymes are typically subject to regulation consisting of trophic hormone stimuli and feedback mechanisms. Very few tissues contribute substantially to de novo steroidogenesis, primarily the adrenal glands, the gonads, and the placenta. Both the embryonic origins and the signaling mechanisms for the adrenals and gonads are similar, and steroid synthesis in these two glands are the major focus of this review. We will further describe peripheral steroid metabolism and the regulation of steroid hormone potency in target tissues. In addition, we will briefly discuss the congenital adrenal hyperplasias to illustrate the principles developed in the initial sections. Finally, we will discuss some recent developments in steroidogensis, focusing on cytochrome P450 oxidoreductase deficiency and the alternate or “backdoor” pathway to dihydrotestosterone. We will conclude with a description of aberrant signaling mechanisms observed in adrenal tumors as a further example of how these pathways can be disturbed in pathologic states.




Similar content being viewed by others
References
Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 1994;269:28314–22.
Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 1996;17:221–44.
Hall PF. Cytochromes P450 and the regulation of steroid synthesis. Steroids 1986;48:133–96.
Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, Gonzalez FJ, Coon MJ, Gunsalus IC, Gotoh O, Okuda K, Nebert DW. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol 1993;12:1–51.
Labrie F. Intracrinology. Mol Cell Endocrinol 1991;78:113–8
Jornvall H, Persson B, Krook M, Atrian S, Gonzalez-Duarte R, Jeffery J, Ghosh D. Short-chain dehydrogenases/reductases (SDR). Biochemistry 1995;34:6003–13.
Jez JM, Penning TM. The aldo-keto reductase (AKR) superfamily: an update. Chem Biol Interact 2001;130–132:499–525.
Rossmann MG, Ford GC, Watson HC, Banaszak LJ. Molecular symmetry of glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol 1972;64:237–45.
Banner DW, Bloomer AC, Petsko GA, Phillips DC, Pogson CI, Wilson IA, Corran PH, Furth AJ, Milman JD, Offord RE, Priddle JD, Waley SG. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 angstrom resolution using amino acid sequence data. Nature 1975;255:609–14.
Simpson ER. Cholesterol side-chain cleavage, cytochrome P450, and the control of steroidogenesis. Mol Cell Endocrinol 1979;13:213–27.
Black SM, Szklarz GD, Harikrishna JA, Lin D, Wolf CR, Miller WL. Regulation of proteins of the cholesterol side-chain cleavage system in Y-1 and JEG-3 cells. Endocrinology 1993;132:539–45.
Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 2002;417:87–91.
Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem 2001;276:46583–96.
Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol 2000;7:408–14.
Miller WL, Strauss JF III. Molecular pathology and mechanism of action of the steroidogenic acute regulartory protein, StAR. J Steroid Biochem Mol Biol 1999;69:131–41.
Lin D, Sugawara T, Strauss JF III, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 1995;267:1828–31.
Caron K, Soo S-C, Wetsel W, Stocco D, Clark B, Parker K. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A 1997;94:11540–5.
Gospodarowicz D, Ill CR, Hornsby PJ, Gill GN. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology 1977;100:1080–9.
Hornsby PJ, Sturek M, Harris SE, Simonian MH. Serum and growth factor requirements for proliferation of human adrenocortical cells in culture: comparison with bovine adrenocortical cells. In Vitro 1983;19:863–9.
Voutilainen R, Miller WL. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain cleavage enzyme, P450scc, in human steroidogenic tissues. Proc Natl Acad Sci U S A 1987;84:1590–4.
Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 1997;18:361–77.
Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994;77:481–90.
Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA, Jameson JL, Sultan C, Dattani MT, Achermann JC. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab 2007;92:991–9.
Prader A, Anders CJPA. Zur Genetik der kongenitalen Lipoidhyperplasie der Nebennieren. Helv Paed Acta 1962;17:285–9.
Lin D, Gitelman SE, Saenger P, Miller WL. Normal genes for the cholesterol side chain cleavage enzyme, P450scc, in congenital lipoid adrenal hyperplasia. J Clin Invest 1991;88:1955–62.
Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia caused by a homozygous frame-shift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab 1997;82:1511–15.
Bose HS, Sugawara T, Strauss JF III, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med 1996;335:1870–8.
Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (P450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab 2001;86:3820–5.
Hiort O, Holterhus PM, Werner R, Marschke C, Hoppe U, Partsch CJ, Riepe FG, Achermann JC, Struve D. Homozygous disruption of P450 side-chain cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. 90:538–41.
Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am 2001;30:101–9.
Biglieri EG, Herron MA, Brust N. 17alpha-hydroxylation deficiency in man. J Clin Invest 1966;15:1945–54.
Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab 2004;89:49–60.
Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20 lyase deficiency. Nature Genet 1997;17:201–5.
Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem 2003;278:48563–9.
Simsek E, Ozdemir I, Lin L, Achermann JC. Isolated 17,20-lyase (desmolase) deficiency in a 46,XX female presenting with delayed puberty. Fertil Steril 2005;83:1548–51.
Bongiovanni AM. The adrenogenital syndrome with deficiency of 3beta-hydroxysteroid dehydrogenase. J Clin Invest 1962;41:2086.
Luu-The V, Lechance Y, Labrie C, Leblanc G, Thomas JL, Strickler RC, Labrie F. Full length cDNA structure and deduced amino acid sequence of human 3beta-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol 1989;3:1310–12.
Rhéaume E, Lachance Y, Zhao HL, Breton N, Dumont M, de Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F. Structure and expression of a new complementary DNA encoding the almost exclusive 3beta-hydroxysteroid dehydrogenase/delta5-delta4-isomerase in human adrenals and gonads. Mol Endocrinol 1991;5:1147–57.
Rhéaume E, Simard J, Morel Y, Mebarki F, Zachmann M, Forest MG, New MI, Labrie F. Congenital adrenal hyperplasia due to point mutations in the type II 3beta-hydroxysteroid dehydrogenase gene. Nature Genet 1992;1:239–45.
Moisan AM, Ricketts ML, Tardy V, Desrochers M, Mebarki F, Chaussain JL, Cabrol S, Raux-Demay MC, Forest MG, Sippell WG, Peter M, Morel Y, Simard J. New insight into the molecular basis of 3beta-hydroxysteroid dehydrogenase deficiency: identification of eight mutations in the HSD3B2 gene in eleven patients from seven new families and comparison of the functional properties of twenty-five mutant enzymes. J Clin Endocrinol Metab 1999;84:4410–25.
Lutfallah C, Wang W, Mason JI, Chang YT, Haider A, Rich B, Castro-Magana M, Copeland KC, David R, Pang S. Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 2002;87:2611–22.
Therrell BLJ, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 1998;101:583–90.
White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 2000;21:245–91.
Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna M, Lesser M, New MI, White PC. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 1992;90:584–95.
White PC, Grossberger D, Onufer BJ, Chaplin DD, New MI, Dupont B, Strominger JL. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A 1985;82:1089–93.
Morel Y, David M, Forest MG, Betuel H, Hauptman G, Andre J, Bertrand J, Miller WL. Gene conversions and rearrangements cause discordance between inheritance of forms of 21-hydroxylase deficiency and HLA types. J Clin Endocrinol Metab 1989;68:592–9.
Speiser PW, New MI, White P. Molecular genetic analysis of nonclassical steroid 21-hydroxylase deficiency associated with HLA-B14DR1. N Engl J Med 1988;319:19–23.
White PC, Curnow KM, Pascoe L. Disorders of steroid 11beta-hydroxylase isozymes. Endocr Rev 1994;15:421–38.
Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases. A new variant of congenital adrenal hyperplasia. N Engl J Med 1985;313:1182–91.
Shackleton C, Malunowicz E. Apparent pregnene hydroxylation deficiency (APHD): seeking the parentage of an orphan metabolome. Steroids 2003;68:707–17.
Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonca BB, Fujieda K, Miller WL. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley–Bixler syndrome. Nat Genet 2004;36:228–30.
Shaw G, Renfree MB, Leihy MW, Shackleton CH, Roitman E, Wilson JD. Prostate formation in a marsupial is mediated by the testicular androgen 5alpha-androstane-3alpha,17beta-diol. Proc Natl Acad Sci U S A 2000;97:12256–9.
Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology 2003;144:575–80.
Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab 2004;15:432–8.
Gupta MK, Guryev OL, Auchus RJ. 5alpha-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys 2003;418:151–60.
Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of a steroid 5alpha-reductase 2 gene in male pseudohermaphroditism. Nature 1991;354:159–61.
Milewich L, Mendonca BB, Arnhold I, Wallace AM, Donaldson MD, Wilson JD, Russell DW. Women with steroid 5alpha-reductase 2 deficiency have normal concentrations of plasma 5alpha-dihydroprogesterone during the luteal phase. J Clin Endocrinol Metab 1995;80:3136–9.
Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med 1996;335:617–23.
Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet 2004;363:2128–35.
Shackleton C, Marcos J, Arlt W, Hauffa BP. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley–Bixler syndrome phenotype. Am J Med Genet A 2004;129:105–12.
Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, Takeda R, Fukami M, Ogata T. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab 2006;91:2643–9.
Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab 2005;90:5446–55.
Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 2004;18:279–90.
Lacroix A, N’Diaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr Rev 2001;22:75–110.
Lacroix A, Bolte E, Tremblay J, Dupre J, Poitras P, Fournier H, Garon J, Garrel D, Bayard F, Taillefer R. Gastric inhibitory polypeptide-dependent cortisol hypersecretion—a new cause of Cushing’s syndrome. N Engl J Med 1992;327:974–80.
Lacroix A, Tremblay J, Rousseau G, Bouvier M, Hamet P. Propranolol therapy for ectopic beta-adrenergic receptors in adrenal Cushing’s syndrome. N Engl J Med 1997;337:1429–34.
Lacroix A, Baldacchino V, Bourdeau I, Hamet P, Tremblay J. Cushing’s syndrome variants secondary to aberrant hormone receptor. Trends Endocrinol Metab 2004;15:375–82.
Mazzuco TL, Chabre O, Feige JJ, Thomas M. Aberrant expression of human luteinizing hormone receptor by adrenocortical cells is sufficient to provoke both hyperplasia and Cushing’s syndrome features. J Clin Endocrinol Metab 2006;91:196–203.
Goodarzi MO, Dawson DW, Li X, Lei Z, Shintaku P, Rao CV, Van Herle AJ. Virilization in bilateral macronodular adrenal hyperplasia controlled by luteinizing hormone. J Clin Endocrinol Metab 2003;88:73–7.
Stratakis CA. Adrenocortical tumors, primary pigmented adrenocortical disease (PPNAD)/Carney complex, and other bilateral hyperplasias: The NIH Studies. Horm Metab Res 2007;39:467–73.
Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the Carney complex. Hum Mol Genet 2000;9:3037–46.
Yamakita N, Murai T, Ito Y, Miura K, Ikeda T, Miyamoto K, Onami S, Yoshida T. Adrenocorticotropin-independent macronodular adrenocortical hyperplasia associated with multiple colon adenomas/carcinomas which showed a point mutation in the APC gene. Intern Med 1997;36:536–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghayee, H.K., Auchus, R.J. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord 8, 289–300 (2007). https://doi.org/10.1007/s11154-007-9052-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-007-9052-2