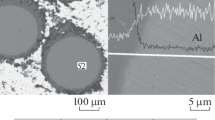
The mechanism of self-propagating high-temperature synthesis (SHS) of AlB2–Al2O3 composite powders was studied using the combustion front quenching method (CFQM). The results showed that the combustion began with fusion of B2O3 and Al particles followed by mutual penetration of Al and B2O3 in the melt. X-ray patterns exhibited reflections for Al2O3 that were consistent with exchange of O atoms between Al and B through the reaction B2O3 + 2Al → 2B + Al2O3. A certain amount of B2O3 volatilized at higher temperatures and reacted with B to form gaseous B2O2. Al2O3 and B precipitated on the Al surface. Then, the produced B dissolved in the Al melt and reacted with Al to precipitate AlB12 particles. Finally, AlB12 transformed into AlB2 at the peritectic temperature during rapid cooling. Thus, the combustion could be explained by a dissolution-precipitation mechanism. The final products included AlB2 and Al2O3 particles and a certain amount of Al. A model of the dissolution-precipitation mechanism was proposed. The ignition temperature of the combustion was ~800°C.













Similar content being viewed by others
References
T. B. Zhu, Y. W. Li, S. B. Sang, and Z. P. Xie, “Formation of nanocarbon structures in MgO–C refractories matrix: Influence of Al and Si additives,” Ceram. Int., 42, 18833 – 18843 (2016).
A. P. Luz, T. M. Souza, C. Pagliosa, M. A. M. Brito, and V. C. Pandolfelli, “In situ hot elastic modulus evolution of MgO–C refractories containing Al, Si or Al–Mg antioxidants,” Ceram. Int., 42, 9836 – 9843 (2016).
J. W. Lian, B. Q. Zhu, X. C. Li, et al., “Effect of in situ synthesized SiC whiskers and mullite phases on the thermo-mechanical properties of Al2O3–SiC–C refractories,” Ceram. Int., 42, 16266 – 16273 (2016).
J. Wu, N. J. Bu, H. B. Li, and Q. Zhen, “Effect of B4C on the properties and microstructure of Al2O3–SiC–C based trough castable refractories,” Ceram. Int., 43, 1402 – 1409 (2017).
W. M. Guo, D.W. Tan, L. Y. Zeng, et al., “Synthesis of fine ZrB2 powders by solid solution of TaB2 and their densification and mechanical properties,” Ceram. Int., (2017).
O. Balci, D. Agaogullari, I. Duman, and M. Lutfi Ovecoglu , “Synthesis of CaB6 powders via mechanochemical reaction of Ca/B2O3 blends,” Powder Technol., 225, 136 – 142 (2012).
H. Sunayama, M. Kawahara, and T. Mitsuo, “Effects of AlB2 addition on the resistance of oxidation of MgO–C refractories,” The PacRim 2nd Refractories Conference, Cairns, Australia, 1996.
J. Chen, K. Chen, Y. G. Liu, et al., “Effect of Al2O3 addition on properties of non-sintered SiC–Si3N4 composite refractory materials,” Int. J. Refract. Met. Hard Mater., 46, 6 – 11 (2014).
V. Muñoz and A. G. Tomba Martinez, “Thermal evolution of Al2O3–MgO–C refractories,” Procedia Mater. Sci., 1, 410 – 417 (2012).
H. S. Tripathi and A. Ghosh, “Spinelisation and properties of Al2O3– MgAl2O4–C refractory: Effect of MgO and Al2O3 reactants,” Ceram. Int., 36, 1189 – 1192 (2010).
L. Zhang, G. Q. Xiao, D. H. Ding, et al., “The effect of Al particle on AlB2–Al2O3 composite powders synthesized by self-propagating high temperature synthesis method,” J. Synth. Cryst. China, 45, 295 – 299 (2016).
H. Q. Yin, G. Q. Xiao, D. H. Ding, et al., “The effect of Mg on the phase composition of AlB2–Al2O3 composite powders synthesized by combustion synthesis,” J. Synth. Cryst. China, 45, 497 – 502 (2016).
E. Sirtl and L. M. Woerner, “Preparation and properties of aluminum diboride single crystals,” J. Cryst. Growth, 16, 215 – 218 (1972).
D. Agaogullari, H. Gokce, I. Duman, and M. Lutfi Ovecoglu, “Aluminum diboride synthesis from elemental powders by mechanical alloying and annealing,” J. Eur. Ceram. Soc., 32, 1457 – 1462 (2011).
A. C. Hall and J. Economy, “Preparing high- and low- aspect ratio AlB2 flakes from borax or boron oxide,” Aluminum Reduction, 2000.
C. Deppisch, et al., “Processing and mechanical properties of AlB2 flake reinforced Al-alloy composite,” Mater. Sci. Eng., A, 225, 153 – 161 (1997).
S. Postrach and J. Potschke, “Pressureless sintering of Al2O3 containing up to 20 vol. % zirconium diboride (ZrB2),” J. Eur. Ceram. Soc., 20, 1459 – 1468 (2000).
L. Li, Y. R. Hong, J. L. Sun, Z. Y. He, and X. Y. Peng, “Formation of ZrB2 in MgO–C-composite materials using in-situ synthesis method,” J. Iron Steel Res. Int., 13(1), 70 – 74 (2006).
G. Merzhanov and I. P. Borovinskaya, “A new class of combustion processes,” Combust. Sci. Technol., 10, 195 – 201 (1975).
H. Q. Che, Y. Ma, and Q. C. Fan, “Investigation of the mechanism of self-propagating high-temperature synthesis of TiNi,” J. Mater. Sci., 46(8), 2437 – 2444 (2011).
Q. C. Fan, H. F. Chai, and Z. H. Jin, “Dissolution-precipitation mechanism of self-propagating high-temperature synthesis of mononickel aluminide,” Intermetallics, 9(7), 609 – 619 (2001).
E. A. Levashova, Yu. S. Pogozhev, A. Yu. Potanin, et al., “Self-propagating high-temperature synthesis of advanced ceramics in the Mo–Si–B system: Kinetics and mechanism of combustion and structure formation,” Ceram. Int., 40, 6541 – 6552 (2014).
A. Mukasyan, A. Pelekh, and A. Varma, “Combustion synthesis in glasses systems under microgravity conditions,” J. Mater. Synth. Process., 5(5), 391 – 400 (1997).
N. Bertolion, U. Anselmi-Tamburini, F. Maglia, G. Spinolo, and Z. A. Munir, “Combustion synthesis of Zr–Si intermetallic compounds,” J. Alloys Compd., 288(1/2), 238 – 248 (1999).
G. Q. Xiao, Q. C. Fan, M. Z. Gu, and Z. H. Jin, “Microstructural evolution during the combustion synthesis of TiC–Al cermet with larger metallic particles,” Mater. Sci. Eng., A, 425(1/2), 318 – 325 (2006).
G. Q. Xiao, Q. C. Fan, M. Z. Gu, Z. H. Wang, and Z. H. Jin, Dissolution-precipitation mechanism of self-propagating high-temperature synthesis of TiC–Ni cermet,” Mater. Sci. Eng., A, 382(1/2), 132 – 140 (2004).
C. L. Yeh and R. F. Li, “Formation of TiB2–Al2O3 and NbB2–Al2O3 composites by combustion synthesis involving thermite reactions,” J. Chem. Eng., 147(1), 405 – 411 (2009).
L. Wang, Z. A. Munir, and J. B. Holt, “Synthesis of Al2O3–B4C composites via a thermite-based combustion reaction,” J. Mater. Synth. Process., 2(4) (1994).
H. C. Yi, J. Y. Guigne, L. A. Robinson, A. R. Manerbino, and J. J. Moore, “Characteristics of porous B4C–Al2O3 composites fabricated by the combustion synthesis technique,” J. Porous Mater., 11(1), 5 – 14 (2004).
S. K. Mishra, S. K. Das, and V. Sherbacov, “Fabrication of Al2O3–ZrB2 in situ composite by SHS dynamic compaction: A novel approach,” Compos. Sci. Technol., 67, 2447 – 2453 (2007).
A. S. Rogachev, A. S. Mukasyan, and A. G. Merzhanov, “Structural transitions during gasless combustion of titanium-boron mixtures,” Dokl. Phys. Chem., 297(6), 1240 – 1243 (1987)].
J. P. Lebrat, A. Varma, and P. J. McGinn, “Mechanistic studies in combustion synthesis of Ni3Al and Ni3Al-matrix composites,” J. Mater. Res., 9(5), 1184 – 1190 (1994).
G. Q. Xiao, Y. L. Fu, Z. W. Zhang, and A. D. Hou, “Mechanism and microstructural evolution of combustion synthesis of ZrB2–Al2O3 composite powders,” Ceram. Int., 41, 5790 – 5797 (2015).
Z. Q. Yu and Z. G. Yang, “ZrB2/Al2O3 composite powders prepared by self-propagating high-temperature synthesis,” Trans. Nonferrous Met. Soc. China, 15(4), 851 – 854 (2005).
S. K. Mishra, S. K. Das, and P. Ramachandrarao, “Self-propagating high-temperature synthesis of a zirconium diboride-alumina composite: a dynamic x-ray diffraction study,” Philos. Mag. Lett., 84(1), 1 – 46 (2004).
D. N. Hendrickson, J. M. Hollander, and W. L. Jolly, “Core-electron binding energies for compounds of boron, carbon, and chromium,” Inorg. Chem., 9(3), 612 – 615 (1970); DOI: https://doi.org/10.1021/ic50085a035.
K. Liu, X. L. Zhou, X. R. Chen, and W. J. Zhu, “Structural and elastic properties of AlB2 compound via first-principles calculations,” Physica B, 388(5), 213 – 218 (2007).
Y. Birol, “Aluminothermic reduction of boron oxide for the manufacture of Al–B alloys,” Mater. Chem. Phys., 136(8), 963 – 966 (2012).
T. Nagai, Ya. Ogasawara, and M. Maeda, “Thermodynamic measurement of (Al2O3 + B2O3) system by double Knudsen cell mass spectrometry,” J. Chem. Thermodyn., 41(11), 1292 – 1296 (2009).
Yu. S. Pogozhev, A. Yu. Potanin, E. A. Levashov, and D. Yu. Kovalev, “The features of combustion and structure formation of ceramic materials in the Cr–Al–Si–B system,” Ceram. Int., 40(7), 16299 – 16308 (2014).
Y. H. Liu, S. Yin, and H. Y. Lai, “Effects of combustion conditions on the characteristics of Al2O3/AlB12 composite powders produced by self-propagation high-temperature synthesis,” J. Inorg. Mater., 15(3), 473 – 479 (2000).
O. N. Carlson, Bull. Alloy Phase Diagrams, 11, No. 6, 560 – 566 (1990).
H. Duschanek and P. Rogl, “The Al–B (aluminum–boron) system,” J. Phase Equilib., 15, 543 (1994).
A. C. Hall, “Pathways to a family of low cast, high performance, metal matrix composites based on AlB2 in aluminum,” The University of Tulsa, 1999.
A. C. Hall and J. Economy, “The Al (l) + AlB12 > AlB2 peritectic transformation and its role in the formation of high aspect ratio AlB2 flakes,” J. Phase Equilib., 21, 63 – 69 (2000).
C. Deppish, G. Liu, A. Hall, et al., “The crystallization and growth of AlB2 single crystal flakes in aluminium,” J. Mater. Res., 13(12), 3485 – 3497 (1998).
O. Savas and R. Kayikci, “A Taguchi optimization for production of Al–B master alloys using boron oxide,” J. Alloys Compd., 580, 232 – 238 (2013).
Acknowledgments
The work was sponsored by the National Natural Science Foundation of China (Grant No. 51272203 and No. 51572212).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 1, pp. 27 – 36, January, 2019.
Rights and permissions
About this article
Cite this article
Yang, P., Xiao, G., Ding, D. et al. Mechanism of Self-Propagating High-Temperature Synthesis of AlB2–Al2O3 Composite Powders. Refract Ind Ceram 60, 46–54 (2019). https://doi.org/10.1007/s11148-019-00307-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-019-00307-z