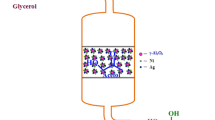
Abstract
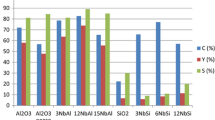
Redox phase unpromoted molybdenum catalysts with different Mo loadings (2.5% Mo/γ-Al2O3; 5.0% Mo/γ-Al2O3; 7.5% Mo/γ-Al2O3 and 10.0% Mo/γ-Al2O3) were prepared and characterized for the ammoxidation of glycerol to nitriles, such as acrylonitrile. The best catalyst (10.0% Mo/γ-Al2O3) obtained a yield of 26% in nitriles. The increase in the molybdenum content and its oxidation states along with the amount of weak/moderate acid sites on the support surface are key points for the optimization of the catalysts.









Similar content being viewed by others
References
Aulakh MK, Pal B (2019) A co-relation study of efficient photocatalytic reduction of aromatic nitriles and band energies of Cu loaded elongated TiO nanocatalysts. J Taiwan Inst Chem Eng 96:559–565. https://doi.org/10.1016/j.jtice.2018.11.009
Ai C, Gong G, Zhao X, Liu P (2017) Macroporous hollow silica microspheres-supported palladium catalyst for selective hydrogenation of nitrile butadiene rubber. J Taiwan Inst Chem Eng 77:250–256. https://doi.org/10.1016/j.jtice.2017.02.031
Rezaie F, Pirouzfar V, Alihosseini A (2020) Technical and economic analysis of acrylonitrile production from polypropylene. Therm Sci Eng Prog 16:100463. https://doi.org/10.1016/j.tsep.2019.100463
Martin A, Kalevaru VN (2010) Heterogeneously catalyzed ammoxidation: a valuable tool for one-step synthesis of nitriles. ChemCatChem 2:1504–1522. https://doi.org/10.1002/cctc.201000173
Zhang Z, Dong B, Zhang Z, Chen J, Xin H, Zhang Q (2020) Separation of acetonitrile + isopropanol azeotropic mixture using ionic liquids with acetate anion as entrainers. Fluid Phase Equilib 521:112725. https://doi.org/10.1016/j.fluid.2020.112725
Galanov SI, Sidorova OI, Gavrilenko MA (2014) The process of acetonitrile synthesis over γ-Al2O3 promoted by phosphoric acid catalysts. Procedia Chem 10:108–113. https://doi.org/10.1016/j.proche.2014.10.020
Liebig C et al (2013) Glycerol conversion to acrylonitrile by consecutive dehydration over WO3/TiO2 and ammoxidation over Sb-(Fe, V)-O. Appl Catal B 132:170–182. https://doi.org/10.1016/j.apcatb.2012.11.035
Pudar S, Oxgaard J, Goddard WA (2010) Mechanism of selective ammoxidation of propene to acrylonitrile on bismuth molybdates from quantum mechanical calculations. J Phys Chem C 114:15678–15694. https://doi.org/10.1021/jp103054x
Cespi D, Passarini F, Neri E, Vassura I, Ciacci L, Cavani F (2014) Life Cycle Assessment comparison of two ways for acrylonitrile production: The SOHIO process and an alternative route using propane. J Clean Prod 69:17–25. https://doi.org/10.1016/j.jclepro.2014.01.057
Brazdil JF (2019) The legacy and promise of heterogeneous selective oxidation and ammoxidation catalysis. Catal Today 363:55–59. https://doi.org/10.1016/j.cattod.2019.04.057
Goyal A (2016) Compositions and methods related to the production of acrylonitrile. https://patents.google.com/patent/US20160368861A1/en. Accessed 26 Jan 2020
Devaux JF and Dubois JL (2016) Process for manufacturing acrolein/acrylic acid. https://patents.google.com/patent/US20130324758A1/en. Accessed 26 Jan 2020
Dubois JL (2010) Method for the synthesis of acrylonitrile from glycerol. https://patents.google.com/patent/US20100048850A1/en. Accessed 26 Jan 2020
Grasselli RK, Trifirò F (2016) Acrylonitrile from biomass: still far from being a sustainable process. Top Catal 59:1651–1658. https://doi.org/10.1007/s11244-016-0679-7
Trade Map - Trade statistics for international business development (2020) ITC - Trade Map. https://www.trademap.org/Index.aspx?AspxAutoDetectCookieSupport=1. Accessed 10 Sept 2020.
Ruy ADS, Alves RMB, Hewer TLR, Pontes DA, Teixeira LSG, Pontes LAM (2020) Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal Today. https://doi.org/10.1016/j.cattod.2020.06.035
Wang Z, Wang L, Jiang Y, Hunger M, Huang J (2014) Cooperativity of Brønsted and Lewis acid sites on zeolite for glycerol dehydration. ACS Catal 4:1144–1147. https://doi.org/10.1021/cs401225k
Guerrero-Pérez MO, Alemany LJ (2008) Alumina supported Mo-V-Te-O catalysts for the ammoxidation of propane to acrylonitrile. Appl Catal A 341:119–126. https://doi.org/10.1016/j.apcata.2008.02.032
Abello MC, Gomez MF, Ferretti O (2001) Mo/γ-Al2O3 catalysts for the oxidative dehydrogenation of propane: effect of Mo loading. Appl Catal A Gen 207:421–431. https://doi.org/10.1016/S0926-860X(00)00680-3
Gadamsetti S, Mathangi N, Hussain S, Velisoju VK, Chary KVR (2018) Vapor phase esterification of levulinic acid catalyzed by γ -Al2O3 supported molybdenum phosphate catalysts. Mol Catal 451:192–199. https://doi.org/10.1016/j.mcat.2018.01.011
Baek M, Lee JK, Kang HJ, Kwon BJ, Lee JH, Song IK (2017) Ammoxidation of propane to acrylonitrile over Mo-V-P-Oy/Al2O3 catalysts: Effect of phosphorus content. Catal Commun 92:27–30. https://doi.org/10.1016/j.catcom.2016.12.022
Braithwaite ER and Haber J (2013) Molybdenum: an outline of its chemistry and use. Elsevier Science
Wang B et al (2012) Effects of MoO3 loading and calcination temperature on the activity of the sulphur-resistant methanation catalyst MoO3/γ-Al2O3. Appl Catal A 431:144–150. https://doi.org/10.1016/j.apcata.2012.04.029
Marakatti VS, Mumbaraddi D, Shanbhag GV, Halgeri AB, Maradur SP (2015) Molybdenum oxide/γ-alumina: an efficient solid acid catalyst for the synthesis of nopol by Prins reaction. RSC Adv 5:93452–93462. https://doi.org/10.1039/c5ra12106j
Thommes M et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:9–10. https://doi.org/10.1515/pac-2014-1117
Han W et al (2020) Selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran catalyzed by ordered mesoporous alumina supported nickel-molybdenum sulfide catalysts. Appl Catal B 268:118748. https://doi.org/10.1016/j.apcatb.2020.118748
Kouachi K, Lafaye G, Pronier S, Bennini L, Menad S (2014) Mo/γ-Al2O3 catalysts for the Biginelli reaction: effect of Mo loading. J Mol Catal A 395:210–216. https://doi.org/10.1016/j.molcata.2014.08.025
Yuan P, Cui C, Han W, Bao X (2016) The preparation of Mo/γ-Al2O3 catalysts with controllable size and morphology via adjusting the metal-support interaction and their hydrodesulfurization performance. Appl Catal A 524:115–125. https://doi.org/10.1016/j.apcata.2016.06.017
Tsukuda E, Sato S, Takahashi R, Sodesawa T (2007) Production of acrolein from glycerol over silica-supported heteropoly acids. Catal Commun 8:1349–1353. https://doi.org/10.1016/j.catcom.2006.12.006
Deleplanque J, Dubois JL, Devaux JF, Ueda W (2010) Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts. Catal Today 157:351–358. https://doi.org/10.1016/j.cattod.2010.04.012
Corma A, Huber GW, Sauvanaud L, O’Connor P (2008) Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J Catal 257:163–171. https://doi.org/10.1016/j.jcat.2008.04.016
Talebian-Kiakalaieh A, Amin NAS, Hezaveh H (2014) Glycerol for renewable acrolein production by catalytic dehydration. Renew Sustain Energy Rev 40:28–59. https://doi.org/10.1016/j.rser.2014.07.168
Possato LG, Diniz RN, Garetto T, Pulcinelli SH, Santilli CV, Martins L (2013) A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J Catal 300:102–112. https://doi.org/10.1016/j.jcat.2013.01.003
Mannei E et al (2017) Light hydrocarbons ammoxidation into acetonitrile over Mo–ZSM-5 catalysts: effect of molybdenum precursor. Micropor Mesopor Mat 241:246–257. https://doi.org/10.1016/j.micromeso.2016.12.021
Jang YH, Goddard WA (2002) Mechanism of selective oxidation and ammoxidation of propene on bismuth molybdates from DFT calculations on model clusters. J Phys Chem B 106:5997–6013. https://doi.org/10.1021/jp0208081
Acknowledgements
This research was financially supported by the Instituto Brasileiro de Tecnologia e Regulação—IBTR, Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq and Fundação de Amparo à Pesquisa do Estado da Bahia—FAPESB.
Author information
Authors and Affiliations
Contributions
Conceptualization: LDS, and LAMP; Methodology: LDS, RCS and JGABS; Software: LDS; Validation: LDS, RCS, JGABS, EPA, RTFF and LAMP; Formal Analysis: LDS, RCS, JGABS and RTFF; Investigation: LDS, RCS and RTFF; Resources: LAMP; Writing – Original Draft Preparation: LDS, RCS, JGABS, EPA, RTFF and LAMP; Writing – Review & Editing: JF: LDS, RCS, JGABS, EPA, RTFF and LAMP; Supervision: LAMP; Project Administration: LAMP; Funding Acquisition: Luiz Antônio Magalhães.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, L.D., Santos, R.C., Silva, J.G.A.B. et al. Direct ammoxidation of glycerol to nitriles using Mo/alumina catalysts. Reac Kinet Mech Cat 135, 271–285 (2022). https://doi.org/10.1007/s11144-021-02111-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02111-8