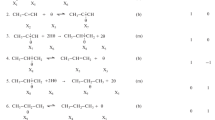Abstract
The palladium-catalyzed phenylacetylene oxidative carbonylation reaction has been shown to produce regular oscillations in pH, reaction heat, and turbidity when conducted in batch with methanol as solvent. Previous studies of the catalytic system showed that water plays an important role in the behavior of the catalytic cycle. Investigating this role further, the reaction was performed in methanol–water mixtures. Experiments demonstrated that, under the conditions studied, oscillations in pH are feasible when the water concentration in the system is increased (0–30 V%). At the same time oscillations visually changed, being transformed from regular oscillations to more stepwise behavior as the proportion of water is increased. By establishing the relationship between the recorded pH and HI concentration it was found that increased water concentration suppresses HI formation and hence slows the autocatalysis in the reaction. It was also found that oscillations begin when the HI concentration is in the region of 1 × 10−3 mol L−1. Additionally, as the amount of water in the system increases a slight decrease in the concentration of HI when oscillations start was noted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbonylation reactions are C–C bond forming reactions that are particularly important for organic synthesis. The carbonylation of phenylacetylene in methanol (Scheme 1), producing the isomeric diesters dimethyl (2Z)-2-phenyl-2-butenedioate and dimethyl (2E)-2-phenyl-2-butenedioate as well as 5,5-dimethoxy-3-phenyl-2(5H)-furanone, has been shown to produce oscillations in pH, redox potential and reaction heat [1,2,3].
The oscillatory nature of changes in pH and reaction heat during the palladium-catalyzed phenylacetylene oxidative carbonylation (PCPOC) reaction has opened this reaction up to new areas of study and possible applications [4, 5]. One of the interesting observations made during previous investigations of the PCPOC reaction at temperatures from 0 to 40 °C was the effect adding methanol to the reaction mixture had on the period and amplitude of the pH oscillations. Without exception, the addition of methanol caused the period and amplitude of the pH oscillations to increase and even appeared to restart oscillations when they had previously ceased [6, 7]. Perturbation studies by Mukherjee found that the addition of HPLC grade methanol to the reaction system when large sustained oscillations were present caused a slight increase in the period of the oscillations, however, when anhydrous methanol was used as both reactant and solvent the reaction did not exhibit any meaningful pH oscillations [8]. As the methanol was in huge excess relative to the other reactants, it is unlikely to be responsible for these observations. The most likely cause is the trace water found in the HPLC methanol (stated as < 0.03% by Sigma-Aldrich) used in the experiments. Water has been shown to affect the behavior of the catalytic system used in the PCPOC reaction and is therefore proposed to be involved in the processes in the reaction mechanism that produce the pH oscillations [9, 10]. Water also reacts in a similar manner to methanol (Scheme 1) leading to the isomeric diacids [(2Z)-2-phenyl-2-butenedioic acid and (2E)-2-phenyl-2-butenedioic acid] and phenylmaleic anhydride [11].
In this study, a series of experiments in which the water concentration was increased from 0 to 40 V% were conducted at 30 °C. This temperature was selected as being within the interval that previously demonstrated oscillatory pH behavior for the PCPOC system. In previously reported results, this temperature gave pH oscillations with a maximum period of 45 min and a reaction time of 3600 min when compared to lower temperature runs that exhibited oscillatory behavior that lasted for several weeks [7, 12, 13]. All experiments were carried out in an HEL Simular reaction calorimeter so that changes in pH and reaction heat (Qr) could be monitored continuously for extended periods of time. To understand the effect of solvent composition on the pH recorded in the experiments, a separate set of titrations was performed to relate the pH measured as a function of hydroiodic acid (HI) concentration as HI is considered to be the reaction product responsible for the pH oscillations. The results of these titrations were used to estimate the concentration of HI that corresponded to the pH recorded in this study.
Experimental
PCPOC experiments
Experiments on the reaction system were conducted using an HEL Simular reaction calorimeter comprising of: a 1 L double jacketed reactor connected to a Julabo heater/circulator; a combined pH electrode; Pt100 temperature probe; a 150 W internal electric heater and 2 mass flow controllers to dose CO and air. All chemicals were purchased from Sigma-Aldrich and used as received. The reaction temperature was set at 30 °C with deionized water volume percent of 0, 5, 20, 30 and 40%. The solvent (approximately 400 mL, with the required composition of Chromasolv HPLC grade methanol (MeOH) and deionized water as shown in Table 1) was added to the reactor.
The solvent mixture was heated to 30 °C while stirring at 250 rpm and palladium(II) iodide (434 mg) was added. The mixture was stirred for 35 min then 114 mg of NaOAc and 37.392 g of KI were added along with approximately 30 mL of the solvent mixture to remove any solids remaining on the reactor walls. The stirrer speed was increased to 350 rpm to increase the rate of KI dissolution. After 10 min, the internal standard naphthalene (1.154 g) was added followed by a further 20 mL of solvent of the required methanol/water composition. The stirrer speed was returned to 250 rpm 5 min after adding the naphthalene. The purging of the reaction mixture with CO (50 mL min−1) and air (50 mL min−1) commenced and continued for the duration of the experiment. Phenylacetylene (6.2 mL) was added after the solution had been purged with CO and air for 20 min. Temperature and pH were monitored throughout each experiment with additional solvent of the required methanol/water composition added as necessary to compensate for evaporative loss.
Determination of relationship between pH measured and HI present in the system
The relationship between pH and HI concentration was established for each solvent composition at 30 °C. A dilute stock solution of HI in methanol was prepared by weighing 50 µL of 57% HI solution in water in a 5 mL volumetric flask and then filling the flask up to the mark with Chromasolv HPLC grade methanol. A 75 mL aliquot of the required methanol/water/KI solution (Table 1) was transferred to a 100 mL conical flask which was subsequently kept in a water bath held at 30 °C for the duration of the experiment. Potassium iodide was included as it is used in a large excess in the PCPOC reactions to dissolve PdI2. This means that the ionic strength of the reaction mixture is high (approximately 0.5 mol L−1). Ionic strength can affect the pH electrode liquid junction potential and the hydrogen ion activity [14, 15]. The large excess of I− will also affect the dissociation of HI. Including KI in the experiments to determine the relationship between the pH measured and the HI present in the system allowed the pH measurements to more accurately reflect those obtained during the PCPOC experiments. The calibrated pH electrode and Pt100 temperature probe from the HEL calorimeter were placed in the flask to monitor pH and temperature. Portions of the dilute HI solution were added gradually to the methanol/water/KI solution starting with 10 µL allowing the pH to stabilize between each addition. After 1 mL of dilute HI solution had been added the 57 wt% HI solution was used to continue the experiment.
Results and Discussion
The pH recorded in the phenylacetylene carbonylation experiments employing HPLC grade methanol and methanol/water mixtures is given in Fig. 1. In the experiment using 100% methanol, the addition of PhAc to the reactor containing the rest of the catalyst components (PdI2, KI and MeOH) resulted in a drop in pH from 7.5 to a pH of 1 before the pH slowly increased and oscillations began. As the amount of water in the system was increased the initial fall in pH took longer, oscillations commenced while pH was decreasing and pH did not recover (Fig. 1).
The pH oscillations for each solvent composition are shown in Figs. 2 and 3. The pH oscillations in Fig. 2 were taken as the baseline pH behavior (100% MeOH) to which the pH behavior in the other solvent compositions could be compared. In 100% MeOH, the pH dropped rapidly after PhAc addition and then rose slowly over the next 1700 min (Fig. 1). When the pH reached 1.5, after 2421 min, oscillations began. Once oscillations started the rate at which the pH rose increased and the pH oscillations gradually increased in period and amplitude (Fig. 2). The rate of the pH increase then slowed at 2900 min until the oscillations stopped.
The trends in the pH oscillations are summarized in Table 2 which shows the amplitude of the oscillations decreased as the amount of water in the system increased. Table 2 also shows that increasing the water volume percent of the system from 5 to 30% delayed the onset of oscillations, with the change from 5 to 20% taking 60% longer before oscillations occur whilst going from 5 to 30% takes almost 3 times longer for oscillations to start. The increase in water content also increased the duration of the oscillations from 1149 min (volume percent 5%) to 3122 min (volume percent 30%).
The pH oscillations for the experiment in which the water volume percent was 5% are shown in Fig. 3a. It shows the onset of pH oscillations occurred when the pH was falling and much earlier (845 min) than in the baseline experiment conducted in 100% MeOH. Initially the shape of the oscillations was similar to that occurring in 100% MeOH (Fig. 2) with a similar period (9 min) and amplitude (0.4 pH units). Note the switching of the oscillatory behavior which occurred after 1245 min producing the large relaxation oscillations (Fig. 3a). This was not due to any deliberate perturbation of the system and was repeatedly noted in experiments under the same initial conditions. This may mark a transition from one oscillatory state to another.
Drastically smaller oscillations were captured in the experiment employing a water volume percent of 20% (Fig. 3b). When a water volume percent of 5% was used, the oscillations occurred when the pH was falling. Oscillations commenced at 1351 min when pH was 4.35 and lasted for 2876 min with pH settling at a pH of approximately 0.08 at the end of the run. In addition to the decrease in size of oscillations, the oscillatory behavior changed to a more stepwise behavior. With a water volume percent of 30%, the oscillatory behavior was similar to that of the experiment in which the water volume percent was 20%. The oscillations still occurred when the pH was falling (at a pH of 4.23), although they took longer to appear (2387 min) and stepwise pH behavior was apparent (Fig. 3c). The maximum period of the oscillations was 25% greater than that observed at a water volume percent of 30% but the amplitude of the oscillations was reduced by almost 67% (Fig. 3b). By the time a water volume percent of 40% was reached, pH oscillations were not discernible (Fig. 3d).
As reactions where conducted in a reaction calorimeter, it was possible to apply power compensation calorimetry to record changes in reaction heat over time [7, 12, 13, 16]. Oscillations in heater power were still evident with 5, 20 and 30% water, were in phase with the pH oscillations and reflected the changes in the pH oscillations as shown in Fig. 4. Note the lack of oscillations in Fig. 4e when the volume percent of water is 40% even though PhAc conversion remained at 95–100% in all experiments.
The synchronization of oscillations in pH and heater power in the PCPOC reaction at 30 °C with water volume percentages of a 0%; b 5%; c 20%; d 30% and e 40%, in which [PhAc] = 0.124 mol dm−3 [PdI2] = 2.64 × 10−3 mol dm−3 [KI] = 0.494 mol dm−3 [NaOAc] = 3.05 × 10−3 mol dm−3 CO = 50 mL min−1 and air = 50 mL min−1
Previous studies of the PCPOC reaction at temperatures from 0 to 40 °C found that changing the reaction temperature produced changes in the shape of the pH oscillations [7, 12]. Increasing the water content of the reaction also affects the shape of the pH oscillations as shown in Table 3.
As the amount of water in the system increased, the oscillations became more step-like in nature (Table 3). When the water volume percent was 5 and 20%, there was also a sudden change near the end of the reaction from small sinusoidal-type oscillations to large oscillations that were almost square. The recorded pH, including the extent of the initial pH drop and the final pH value was dependent on the amount of water in the system—once water was in the system, increasing water content reduced the size of the initial pH drop and the final pH reading. While pH clearly differs between runs, as the measurement of pH is affected by the composition of the solvent it is difficult to meaningfully compare oscillatory characteristics such as the amplitude of the oscillations between these experiments. This is a consequence of the fact that the standard pH scale is based on measurements in water. As the amount of organic solvent in the system is increased, it alters the pKa of the acid in the system and hence alters the pH that is observed. It has been reported that in 100% methanol pH values can be adjusted by adding 2.3 to the observed pH measurements to get the equivalent pH value in water [17]. However, as the solvent composition changes, the amount by which the observed pH values need to be adjusted also changes [18]. This means that under the conditions used in these experiments a direct comparison of the observed pH values is challenging. To overcome this issue, the relationship between HI concentration and pH in the methanol/water/KI systems used in the reactions was determined by adding HI to methanol/water/KI solutions of the required compositions and measuring the pH. Fig. 5 shows the relationship obtained for each solution.
Employing the data shown in Fig. 5, the best fit curve for each solution composition over the required pH range was obtained (Table 4) and used to estimate the concentration of HI produced during the PCPOC experiments from the pH recorded (Fig. 1). The estimated concentrations of HI in each of the PCPOC experiments are shown in Fig. 6.
A summary of the trends in the oscillations in HI concentrations in the PCPOC reactions is given in Table 5. In 100% methanol the initial stage of the reaction is characterized by a large increase in HI concentration to a maximum of 5.1 × 10−3 mol L−1. Oscillations are only apparent once this large concentration has decreased to 1.43 × 10−3 mol L−1. The relatively large pH oscillations (0.6 pH units, Table 2 and Fig. 2) recorded in 100% methanol are actually small oscillations in the concentration of HI with a maximum amplitude of 1.78 × 10 −4 mol L−1 (Fig. 6; Table 5). It is interesting to note that oscillations only commence once the HI concentration is in the region of 1 × 10−3 mol L−1. As the amount of water in the system increases the concentration of HI when oscillations start reduces. Once water is present in the system it suppresses the initial formation of HI, resulting in a slower autocatalytic process.
Conclusions
This study has found that oscillations in pH and reaction heat are still observable when the volume of water in the PCPOC reaction is increased up to 30% water. On the strength of this finding, optimization of the reaction conditions in mixtures with water would be beneficial in order to further the development of applications of this reaction, for example, using polymeric substrates in this reaction for medical applications. Solvent composition affects the pH measurement so direct comparison of the pH in the different solvent compositions is not meaningful. However, by establishing the relationship between HI concentration and the observed pH at each solvent composition it has been possible to estimate the change in concentration of HI during the reaction. This has shown that, under the initial conditions studied, as the concentration of water in the system increases HI formation is suppressed until, at 40% water, oscillations cease. It has also shown that oscillations commence when the HI concentration is in the region of 1 × 10−3 mol L−1, with this value slightly decreasing as water concentration increases.
References
Gorodskii SN, Kalenova ES, Bruk LG, Temkin ON (2003) Oxidative carbonylation of alkynes in self-oscillating mode. Effect of the nature of substrates on the dynamic behavior of reaction system. Russ Chem Bull 52(7):1534–1543
Gorodskii SN, Zakharov AN, Kulik AV, Bruk LG, Temkin ON (2001) Oxidative carbonylation of alkynes in an oscillation mode: I. Concentration limits for oscillations in the course of phenylacetylene carbonylation and possible mechanisms of the process. Kinet Catal 42(2):251–263
Malashkevich AV, Bruk LG, Temkin ON (1997) New oscillating reaction in catalysis by metal complexes: a mechanism of alkyne oxidative carbonylation. J Phys Chem A 101(51):9825–9827
Isakova A, Novakovic K (2017) Oscillatory chemical reactions in the quest for rhythmic motion of smart materials. Eur Polym J. doi:10.1016/j.eurpolymj.2017.08.033
Donlon L, Novakovic K (2014) Oscillatory carbonylation using alkyne-functionalised poly(ethylene glycol). Chem Commun 50(98):15506–15508
Parker J (2016) A study of the phenylacetylene oxidative carbonylation reaction in oscillatory and non-oscillatory modes. Doctoral Thesis, Newcastle University, Newcastle Upon Tyne, UK, Newcastle University, Newcastle Upon Tyne, UK
Parker J, Novakovic K (2016) Autonomous reorganization of the oscillatory phase in the PdI2 catalyzed phenylacetylene carbonylation reaction. React Kinet Mech Cat 118(1):73–85
Mukherjee A (2009) Analysis and control of oscillations and chaos in reactions. PhD, Newcastle University
Parker J, Novakovic K (2013) Influence of water and the reactant addition sequence on palladium(II) iodide-catalyzed phenylacetylene carbonylation. Ind Eng Chem Res 52(7):2520–2527
Novakovic K, Parker J (2011) Catalyst initiation in the oscillatory carbonylation reaction. Int J Chem Eng. doi:10.1155/2011/518592
Gabriele B, Costa M, Salerno G, Chiusoli GP (1994) An efficient and selective palladium-catalysed oxidative dicarbonylation of alkynes to alkyl- or aryl-maleic esters. J Chem Soc Perkin Trans 1:83–87
Novakovic K, Mukherjee A, Willis MJ, Wright AR, Scott SK (2009) The influence of reaction temperature on the oscillatory behaviour in the palladium-catalysed phenylacetylene oxidative carbonylation reaction. PCCP 11:9044–9049
Parker J, Novakovic K (2017) The effect of temperature on selectivity in the oscillatory mode of the phenylacetylene oxidative carbonylation reaction. ChemPhysChem 18:1981–1986
Brandariz I, Barriada JL, Taboada-Pan C, de Vicente MES (2001) Estimating the change in liquid junction potential on glass electrodes. Electroanalysis 13(13):1110–1114
Brandariz I, Vilariño T, Alonso P, Herrero R, Fiol S, Sastre de Vicente ME (1998) Effect of ionic strength on the formal potential of the glass electrode in various saline media. Talanta 46(6):1469–1477
Novakovic K, Grosjean C, Scott SK, Whiting A, Willis MJ, Wright AR (2007) Achieving pH and Qr oscillations in a palladium-catalysed phenylacetylene oxidative carbonylation reaction using an automated reactor system. Chem Phys Lett 435(1–3):142–147
Porras SP, Kenndler E (2004) Kenndler E (2004) Capillary zone electrophoresis in non-aqueous solutions: pH of the background electrolyte. J Chromatogr A 1037(1–2):455–465
Rosés M (2004) Determination of the pH of binary mobile phases for reversed-phase liquid chromatography. J Chromatogr A 1037(1–2):283–298
Acknowledgements
The authors wish to acknowledge the UK Engineering and Physical Sciences Research Council (EPSRC) Grant no. EP/H003908/1 for funding this research. Dr Julie Parker would like to acknowledge Professor Allen Wright for funding her Ph.D. studies. Data supporting this publication is openly available under an ‘Open Data Commons Open Database License’. Additional metadata are available at: http://dx.doi.org/10.17634/082584-2. Please contact Newcastle Research Data Service at rdm@ncl.ac.uk for access instructions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Parker, J., Novakovic, K. The effect of using a methanol–water solvent mixture on pH oscillations in the palladium-catalyzed phenylacetylene oxidative carbonylation reaction. Reac Kinet Mech Cat 123, 113–124 (2018). https://doi.org/10.1007/s11144-017-1282-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1282-z