Abstract
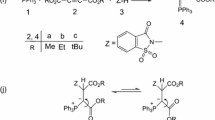
Quantum mechanical calculations were used to clarify how the phosphorus ylides exist as a mixture of the two geometrical isomers (Z- and E-) as a major or minor form. In addition, kinetic studies were made for the reaction between triphenylphosphine and di-alkyl acetylenedicarboxylates in the presence of protic/nucleophilic reagent, such as pyridazinone. To determine kinetic parameters, the reaction was monitored by UV spectrophotometry. The values of the second order rate constant (k 2) were calculated using standard equations. Useful information was obtained from studies of the solvent effect, the structure of reactants (different alkyl groups within the structure of dialkyl acetylenedicarboxylates) and also the concentration of reactants on the rate of reaction. The proposed mechanism was confirmed according to the obtained results and steady state approximation. The first (k 2) and third (k 3) steps of reactions were recognized on the basis of experimental data as the rate determining and fast steps, respectively.





Similar content being viewed by others
References
Crayson M, Griffith EJ (1972) Topics in phosphorus chemistry. Insterscience, New York
Sikorski JA, Logusch EW (1992) Aliphatic carbon-phosphorus compounds as herbicides. Handbook of organophosphorus chemistry. Marcel Dekker, New York
Maghsoodlou MT, Habibi-Khorassani SM, Heydari R, Hassankhani A, Marandi G, Nassiri M, Mosaddege E (2007) Synthesis of oxime phosphoranes from reaction between triphenylphosphine and acetylenic esters in the presence of oxime derivatives. Mol Divers 11:87–91
Shaabani A, Soleimani E (2007) Synthesis a novel class of unsaturated cyclic compounds containing phosphorus atom using pseudo four-component condensation reactions. J Iran Chem Soc 4:497–0502
Heydari A, Arefi A (2007) One-pot three-component synthesis of α-amino phosphonate derivatives. Catal Commun 8:1023–1026
Maghsoodlou MT, Heydari R, Hazeri N, Habibi-Khorassani SM, Nassiri M, Kazemian MA, Salehzadeh J, Hajizadeh M, Ghasemzadeh M, Mosaddegh E (2008) Synthesis of heterocyclic stable phosphorus ylides from reaction between triphenylphosphine and activated acetylenic esters in the presence of biological active NH heterocyclic compounds. Biomed Pharmacol 1:51–60
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–741
Frisch MJ, Trucks GW, Schlegel HB et al (2003) Gaussian 03, Revision B.05. Gaussian Inc, Pittsburgh
Bader RFW (1990) Atoms in molecules: A quantum theory. Oxford University, Oxford
Biegler-konig F, Schonbohm J (2001) Update of the AIM2000-program for atoms in molecules. J Comput Chem 22:1489–1494
Grabowski SJ (2001) J Mol Struct 562:137
Arnold WD, Oldfield E (2000) The chemical nature of hydrogen bonding in proteins via NMR: J-couplings, chemical shifts, and AIM theory. J Am Chem Soc 122:12835–12841
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:1115–11161
Schwartz LM, Gelb RI (1978) Alternative method of analyzing first-order kinetic data. Anal Chem 50:1592–1594
Okubo T, Maeda Y (1989) Kitano, H. Inclusion process of ionic detergents with cyclodextrins as studied by the conductance stopped-flow method. J Phys Chem 93:3721–3723
Acknowledgments
The authors sincerely thank Payam Noor University, the University of Sistan and Baluchestan and Sirjan University of Technology for providing financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zakarianezhad, M., Habibi-Khorassani, M., Khajehali, Z. et al. Mechanistic investigation of the reaction between triphenylphosphine, dialkyl acetylenedicarboxylates and pyridazinone: a theoretical, NMR and kinetic study. Reac Kinet Mech Cat 111, 461–474 (2014). https://doi.org/10.1007/s11144-013-0653-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0653-3