Abstract
This paper demonstrates the benefit of using a ternary Cu–Cr–Al system as a support for palladium catalysts in the methanol synthesis reaction (MS) obtained from CO hydrogenation. The influence of ternary oxide composition and palladium addition on the physicochemical and catalytic properties of Pd/Cu–Cr–Al catalysts in methanol synthesis is discussed. The physicochemical properties of the studied systems were examined by XRD, TPR and BET methods. The promotion effect of palladium on the catalytic activity and reduction of ternary oxide was proven. It was found that palladium promoted catalysts prepared from palladium nitrate precursor showed higher yield in methanol formation.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Methanol is a top chemical worldwide, which can be used as a fuel, constituent of fuel, intermediate to produce a wide range of chemicals and finally as a laboratory solvent [1]. The growing interest of the methanol production in recent years is apparent from the use of this material as a source of hydrogen to power fuel cells, especially in the case of direct-methanol fuel cell technology [2–4]. The main advantage of methanol as a hydrogen carrier is a high hydrogen-to-carbon ratio, molecular simplicity (no C–C bond), relatively low reforming temperatures (250–350 °C) due to its low energy chemical bond, and low sulfur content (<0.5 ppm) [5, 6].
Methanol is conventionally obtained from synthesis gas (mixture of CO and H2) originating from renewable (biomass) and non-renewable (fossil fuels) feedstock’s [1]. The annual output of methanol reached 30 million tons [7] and it is still growing. Currently, methanol is obtained from syngas at the temperature range 200–250 °C under the pressure of 0.5–10 MPa, according to the following reactions:
Methanol production from syngas has been carried out since the 1960s over Cu/ZnO catalyst modified by chromium or/and aluminum oxides. Copper based catalysts are commonly used in many chemical reactions such as the water gas shift reaction (WGS), methanol steam reforming, methanol decomposition or NO reduction. Catalytic systems based on copper oxide are characterized with high selectivity in methanol formation [8, 9]. The improvement of copper catalyst stability is achieved by the modification of CuO by Cr2O3 addition [9].
Investigation of the Cr2O3–CuO/Al2O3 catalysts in the methanol synthesis reaction proved that increasing of Cu and Cr loading on catalyst surface leads to an improvement of catalyst activity and stability in spite of specific surface area decreasing for studied catalytic system [10].
Catalysts for methanol synthesis have been modified not only by implementing a new component such as metal oxides (for instance chromium oxide), but also through promotion by a noble metal. A large amount of research was carried out to study the impact of noble metals Pd [11, 12], Pt [11, 12], Au [13, 14], Ag [13, 15] on the catalytic activity of copper catalysts. The most suitable promoter of copper catalysts was found to be palladium, which improves the catalytic activity and selectivity of copper catalysts significantly [11, 12].
Kugai et. al. [11] confirmed improvement of catalytic efficiency in the case of catalysts promoted by palladium in WGS reaction.
Epling et al. [16] tested palladium catalysts in higher alcohol synthesis and they demonstrated that Pd addition increases the total alcohol production rate of Zn/Cr-spinel based catalysts.
The aim of this work was to find the impact of Pd addition (regarding to Pd salt precursor Pd(NO3)2 and PdCl2) and the effect of ternary oxide Cu–Cr–Al composition on the physicochemical properties and catalytic activity in the methanol synthesis reaction. In this study, 5 %Pd/xCuO:yCr2O3:zAl2O3 catalysts were prepared by the wet impregnation method and characterized by the nitrogen adsorption method (BET), temperature programmed reduction (TPR-H2) and X-ray diffraction (XRD) techniques. Catalytic activity tests in the hydrogenation of carbon monoxide were performed in the methanol synthesis reaction at two temperatures 260 and 290 °C under elevated pressure (3 MPa).
Experimental
Catalysts preparation
The nitrates of copper, chromium and aluminum were used as support material precursors. Ternary systems were prepared by the co-precipitation method of appropriate hydroxides using ammonia hydroxide as a precipitating agent. The mixtures were dried (T = 100 °C for 24 h) and calcined 3.5 h in air atmosphere at various temperatures 400, 700 and 900 °C. Appropriate amounts of copper, chromium and aluminum nitrates were taken to lead to formulas of ternary system with following molar ratios of oxides CuO:Cr2O3:Al2O3 like 1:0.5:1, 0.5:0.5:1, 0.25:0.5:1, 1:2:1. Palladium catalysts were prepared by the wet impregnation method using aqueous solutions of palladium chloride and nitrate. The Pd loading in all catalytic systems was 5 wt% Pd
The specific surface area (BET)
The specific surface area and porosity for catalysts and ternary systems were determined with an automatic sorptometer Sorptomatic 1900. Samples were prepared at 250 °C during a 12-h evacuation and after that, low temperature nitrogen adsorption–desorption measurements were carried out.
Temperature programmed reduction (TPR-H2)
The TPR-H2 measurements were carried out in automatic TPR system AMI-1 in temperature range 25–900 °C with the linear heating rate 10 °C/min. Samples (weight around 0.1 g) were reduced in a hydrogen stream (5 %H2–95 %Ar) with the gas volume velocity 40 cm3/min. Hydrogen consumption was monitored by a thermal conductivity detector (TCD).
Phase composition studies–XRD measurements
Room temperature powder X-ray diffraction patterns were collected using a PANalytical X’Pert Pro MPD diffractometer in Bragg–Brentano reflecting geometry. Copper CuKα radiation from a sealed tube was utilized. Data were collected in the range 5–90° 2θ with step 0.0167° and exposition per one step of 27 s. Due to the fact that raw diffraction data contain some noise, the background during the analysis was subtracted using the Sonneveld, E. J. and Visser algorithm and next the data were smoothed using cubic polynomial. All calculations were done with X’Pert HighScore Plus computer program.
Catalytic activity tests
Activity tests in the methanol synthesis reaction were carried out using the high pressure fixed bed reactor using a gas mixture of H2 and CO with molar ratio 2:1. The process was carried out under elevated pressure (3 MPa) at 260 and 290 °C and products were analyzed by gas chromatography. Before activity tests, all catalysts were pre-reduced for 2 h in a flow of 5 %H2–95 %Ar mixture at 300 °C under atmospheric pressure. The steady-state activity measurements were taken after at least 12 h on the stream.
Results and discussion
The specific surface area (BET)
The results of specific surface area measurements of CuO:Cr2O3:Al2O3 catalysts described by molar ratios x:y:z = 1:0.5:1, 0.5:0.5:1, 0.25:0.5:1, 1:2:1 as well as catalysts promoted by Pd obtained by impregnation using two types of precursors (palladium chloride or nitrate) are shown in Table 1. The obtained data for unpromoted copper catalysts calcined at 400 °C had the specific surface area in the range of 181–236 m2/g. An increase of the calcination temperature from 400 to 900 °C causes a decrease in the surface area up to 10–17 m2/g. The decrease of the specific surface area can be explained by sintering that occurs at higher temperature and by the formation of crystal structures during calcination process. The spinel structure CuCr2O4, CuAl2O4 (see Figs. 1, 2, 3) crystal phases formed during the calcination process carried out at 700 and 900 °C are all characterized by a low value of specific surface area what explain the observed results. The presence of the above-mentioned spinel compounds were confirmed by XRD measurements (see Figs.1, 2, 3).
The copper catalysts systems doped by Pd calcined at 400 °C showed only slight decrease in the value of specific surface area compared to undoped catalysts. The influence of the kind of Pd precursor on the specific surface area was also found after specific surface area measurements.
A more pronounced decrease in the value of the specific surface area ranging from 5 to 8.5 % was detected for catalysts impregnated by PdCl2 containing in their composition ternary system with various molar ratios of the oxides CuO:Cr2O3:Al2O3 like 0.5:0.5:1, 0.25:0.5:1 and 1:2:1 (see Table 1). This result could be explain by the formation of thermally stable (at 400 °C) CuCl2 during calcination process, which fills the pores of the ternary oxide. In the case of palladium doped catalysts obtained from palladium nitrate precursor, no significant effect on the S BET was observed.
Generally, the results of the specific surface area measurements clearly show that the increase of calcination temperature causes a drop in the value of specific surface area related to the phase transformation of catalytic systems and sintering process. Only a slight decrease of the surface area for catalysts impregnated by Pd precursors was confirmed.
Phase composition studies
The phase composition studies were performed for all studied catalysts to understand the interaction between all component systems. The X-ray patterns of undoped and promoted by Pd (impregnated from PdCl2 or Pd(NO3)2 solution) ternary catalytic systems like xCuO:yCr2O3:zAl2O3 after calcination in air at 400, 700 and 900 °C are shown in Figs. 1, 2 and 3.
Fig. 1 presents the phase composition studies of ternary catalysts with various molar ratios of the corresponding oxides xCuO:yCr2O3:zAl2O3. The XRD patterns recorded for all samples calcined at 400 °C confirmed the presence of poorly crystalline: γ-Al2O3 (2θ = 37.6°, 39.5° 45.9°, 67.0° [ICDD PDF-2 WIN 00-010-0425]) and CuO (2θ = 35.5°, 38.7°, 39.0°, 48.7° [ICDD PDF-2 WIN 00-045-0937]) phases. The increase of the calcination temperature from 400 to 700 and 900 °C causes the growth of crystallization degree in all cases. Additionally, the calcination process carried out at 700 and 900 °C, for all catalysts, leads to spinel structure formation describing by following formula CuAl2O4 (2θ = 31.3°, 36.9°, 44.9°, 55.8°, 59.4°, 65.3°, 74.2°, 77.5° [ICDD PDF-2 WIN 00-033-0448]) and a new crystalline phase Al2Cu2Cr2O8 (2θ = 31.4°, 36.5°, 56.0° [ICDD PDF-2 WIN 00-052-1128]) irrespective of the ternary oxide composition.
The phase composition and reduction behavior of CuO/Al2O3 catalyst was studied in an earlier work [17]. The authors reported that the spinel structure CuAl2O4 was formed during the calcination of copper catalyst at 700 °C. The intensity of copper aluminate increases together with the growth of CuO loading to 20 wt% of CuO. A further increase of CuO loading causes that the intensity of CuAl2O4 diffraction peak is closed to that of 20.0 %. This indicates that a part of CuO changes into CuAl2O4 via solid–solid interaction with Al2O3 [18]. A large amount of CuAl2O4 spinel structure on the catalyst surface inhibits CuO diffusing into Al2O3 support.
It is worth emphasizing that only in the case of ternary systems 0.25CuO:0.5Cr2O3:Al2O3 and CuO:2Cr2O3:Al2O3, an extra spinel CuCr2O4 phase (2θ = 35.2, 37.7, 42.3, 56.2, 64.8 [ICDD PDF-2 WIN 00-034-0424]) was also formed during the calcination process (see Fig. 1C, D). The explanation of this result is the composition of these systems, in which one atom of copper is attributable on two atoms of chromium, which facilitates the formation of this spinel CuCr2O4 structure. XRD results obtained for ternary oxide clearly showed that the intensity of copper aluminate increases together with increasing alumina content in studied system. The same tendency is observed for CuCr2O4 structure, the intensity of its reflexes increases with an increasing content of copper and chromium in the ternary system.
Amin et. al. [19] investigated the phase composition of copper–chromium(III) catalysts and they also confirmed the formation of copper chromite spinel-like structure (CuCr2O4) and small quantities of α-Cr2O3 [20]. In our previous works [13, 14, 21], we obtained similar results. We studied the phase composition of copper supported catalysts calcined in air at 400 °C and reported that copper catalysts were a mixture of CuO, α-Cr2O3, CuCr2O4 and amorphous Al2O3 phases.
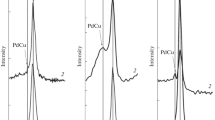
The phase composition studies carried out for palladium promoted catalysts prepared by the impregnation method using PdCl2 and Pd(NO3)2 precursor are shown in Figs. 2 and 3, respectively. The XRD patterns of palladium catalysts prepared from chloride palladium precursor containing various compositions of ternary systems are given in Fig. 2. The XRD curves of catalysts calcined at 400 °C showed the same phases γ-Al2O3 and CuO as previously observed for ternary systems. The increase of calcination temperature to 700 and 900 °C caused the growth of crystallization degree in the case of all catalysts. Additionally, the same phases γ-Al2O3, CuO, CuAl2O4, CuCr2O4, Al2Cu2Cr2O8 were visible on the XRD patterns depending on catalysts composition as in the case of ternary systems described previously. The only differences stemmed from the palladium oxide phase for 2θ = 33.8, 54.7, 60.2.
The study of the phase compositions of palladium catalysts obtained from the palladium nitrate (see Fig. 3) precursor showed the same tendency as for above-described for doped catalytic systems. For palladium catalysts calcined at 400 °C, the presence of γ-Al2O3 and CuO phases were also confirmed. The presence of following reflections originating from appreciate crystallographic phases: γ-Al2O3, CuO, PdO, CuAl2O4, Al2Cu2Cr2O8 were confirmed for palladium catalysts calcined at 700 and 900 °C containing in their composition following ternary systems: CuO:0.5Cr2O3:Al2O3 and 0.5CuO:0.5Cr2O3:Al2O3. For the 5 %Pd/0.25CuO:0.5Cr2O3:Al2O3 and 5 %Pd/CuO:2Cr2O3:Al2O3 catalysts calcined at 700 and 900 °C, we observed an additional CuCr2O4 spinel structure for 2θ = 35.2, 37.7, 42.3, 56.2, 64.8 [ICDD PDF-2 WIN 00-034-0424] in addition to γ-Al2O3, CuO, PdO, CuAl2O4, Al2Cu2Cr2O8 phases (in the same 2θ values as mentioned before) in the XRD patterns.
The formation of spinel structure compounds was caused by solid–solid interactions of CuO with γ-Al2O3, and CuO with α-Cr2O3 at temperatures above 700 °C during the calcination process. In our previous work, the phase composition studies of calcined monometallic copper catalysts supported on CrAl3O6 or spinel-like structure ZnAl2O4 confirmed the formation of copper chromite [13, 14] and copper aluminate [8, 22] spinel-like structure depending on the catalyst composition.
Reduction studies
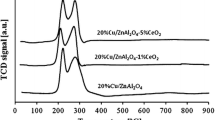
The reduction studies were performed for ternary and promoted ternary catalysts to explore individual component interactions. For a better understanding of the reduction behavior of investigated catalysts, we studied the TPR of reference materials CuCr2O4, CrO3, PdO/Al2O3, CuO/Al2O3 and CuAl2O4 in the first stage of our measurements. The profiles of temperature programmed reduction for reference compounds are presented in Fig. 4A and B.
Fig. 4A presents the reduction profiles of the CuCr2O4, CrO3 and PdO/Al2O3 systems. The TPR curve of CuCr2O4 exhibited only one reduction stage with the maximum of hydrogen consumption peak situated at 250 °C, which is attributed to the copper–chromium(III) reduction. The next presented profile is the reduction of the CrO3 system. One can easily observe that CrO3 [13, 14, 21] is reduced in one stage connected with the reduction of Cr(VI) surface species to Cr(III) species (see Fig. 4A). The maximum of this reduction effect is located at 380 °C.
The last profile visible in Fig. 4A presents the reduction process of the PdO/Al2O3 catalyst. The behavior of this profile confirms the one step reduction process of palladium catalyst. This stage is attributed to PdO reduction, which takes place in the low temperature range 60–110 °C.
Cubeiro and Fierro [23] studied the reduction behavior of palladium supported catalysts and they claimed that PdO is an easily reducible oxide, even at room temperature. Authors also observed a low temperature reduction profile situated in the temperature range 30–150 °C, which was assigned to the reduction of palladium oxide to metallic palladium.
We also studied the reduction behavior of palladium catalysts supported on spinel structure (2 %Pd/ZnAl2O4) and we observed two hydrogen consumption peaks situated at about 30 and 80 °C. The first hydrogen consumption peak was attributed to the reduction of highly dispersed palladium oxide, which is slowly reduced already at room temperatures. The second reduction profile was assigned to the reduction of small palladium crystallites interacting with the surface of ZnAl2O4 [24].
Fig. 4B shows the reduction of CuO/Al2O3 and CuAl2O4 catalysts. Those reduction measurements were performed to understand the interaction between CuO and Al2O3 compounds. The reduction profile of CuO/Al2O3 supported catalysts directly indicates that reduction of copper(II) oxide runs in one step, and this stage includes the reduction of CuO to metallic copper which takes place in the temperature range 210–350 °C. Whereas the reduction of CuAl2O4 runs in three inseparable reduction stages (see Fig. 4B). The first observed reduction effect is attributed to the reduction of CuO, next profile present copper oxide reduction being with interaction with alumina network and finally last peak present hard reduced copper aluminate spinel structure reduction [8, 22].
Reduction measurements of ternary oxides xCuO:yCr2O3:zAl2O3 and palladium catalysts prepared using the chloride or nitrate palladium precursor are given on Figs. 4C, 4D, 4E, and 4F. The TPR profiles of ternary oxides CuO:0.5Cr2O3:Al2O3, 0.5CuO:0.5Cr2O3:Al2O3 and 0.25CuO:0.5Cr2O3:Al2O3 consist of one wide reduction effect which ends at about 300 °C. This wide reduction stage represent the reduction of CuO, CuCr2O4 and Cr(VI) surface species according to reactions 1, 2 and 3 (listed later).
Only in the case of the CuO:2Cr2O3:Al2O3 ternary oxide, which contains the highest concentration of Cr2O3, the TPR profile showed two partially resolved reduction stages. The first reduction effect is connected with CuO and CuCr2O4 reduction. The maximum of the second reduction peak situated at 330 °C is attributed to a Cr(VI) oxidized phase formed from the previously reoxidized phase of Cr2O3. The rest of Cr2O3 compound create the CuCr2O4 phase undergo the reduction process in the first stage of reduction.
It is worth noting that the different shape of TPR profiles recorded for ternary oxide is a function of various molar ratios of appreciate oxide xCuO:yCr2O3:zAl2O3 catalysts. That is so only in the case of CuO:2Cr2O3:Al2O3 system, which contains the highest wt% (62.4 %) of Cr2O3. Here, two hydrogen consumption peaks are easily visible.
The TPR profiles of 5 %Pd/CuO:0.5Cr2O3:Al2O3, 5 %Pd/0.5CuO:0.5Cr2O3:Al2O3 and 5 %Pd/0.25CuO:0.5Cr2O3:Al2O3 (see Figs. 4C, 4D, and 4E) catalysts prepared from PdCl2 precursor present a two-step reduction (see Figs††. 4A, 4B, 4C, and 4D). The addition of palladium to ternary systems facilitates the reduction process of the CuO:Cr2O3:Al2O3 ternary oxide. The first effect presents the reduction of PdO according to reaction 5. The second profile is connected with CuO, CuCr2O4 and Cr(VI) surface species reduction. Differences were only observed for the 5 %Pd/CuO:2Cr2O3:Al2O system, which undergoes reduction in one unresolved wide peak. The shape of this profile suggested that it presents the reduction of the same compounds that were observed in the case of above-described systems.
The results of TPR measurements for palladium catalysts prepared by the impregnation of ternary oxide xCuO:yCr2O3:zAl2O3 using palladium nitrate precursor showed mainly one reduction effect situated in the temperature range 100–280 °C (see Figsboth palladium chloride. 4C, 4E, and 4F). One reduction effect was observed for the 5 %Pd/CuO:0.5Cr2O3:Al2O3, 5 %Pd/0.25CuO:0.5Cr2O3:Al2O3 and 5 %Pd/0.5CuO:0.5Cr2O3:Al2O3 catalyst and the position of the maximum of hydrogen consumption peak in all cases were shifted into the lower temperature range. In those cases, the shift of the reduction peaks also confirm that the addition of palladium facilitates the reduction of ternary oxides xCuO:yCr2O3:zAl2O3. This fact can be explained by the spill-over effect between palladium and remaining oxide (CuO, CuCr2O4 and Cr(VI) surface species) forms, which are reduced during reduction process. The TPR reduction profile of the 5 %Pd/0.5CuO:0.5Cr2O3:Al2O3 catalyst exhibited two unresolved reduction effects connected with the same oxide forms reduction as for the above-described catalytic systems (see Fig. 4D).
Moreover, hydrogen consumption peaks for catalysts impregnated by Pd(NO3)2 show one peak, while after PdCl2 impregnation, two separate peaks were detected. The observed result can be caused by Pd–O–Cl interactions on the catalyst surface in the case of palladium catalysts obtained after the impregnation of the ternary oxide by palladium chloride precursor.
Catalytic activity tests
Activity measurements were determined for the selected ternary oxide (xCuO:yCr2O3:zAl2O3) and palladium catalysts at two reaction temperatures (260 and 290 °C). Before the activity tests, all catalytic systems were activated by the reduction at 300 °C for 2 h in a reduction mixture 5 %H2–95 %Ar under atmospheric pressure. The reaction was carried out from CO/H2 mixture with molar ratio CO to H2 equal to 0.5 under the elevated pressure (3 MPa). The results of the activity tests are presented in Fig. 5A, B, C.
Catalytic activity measurements show generally one common correlation, that the improvement of methanol yield was observed together with the increase of reaction temperature from 260 to 290 °C for all ternary and palladium ternary catalysts (from both palladium chloride (Fig. 5B) or palladium nitrate (Fig. 5C) precursor).
Fig. 5 clearly shows that the palladium catalysts had the highest activity in the methanol synthesis reaction. The activity of palladium catalysts impregnated by Pd solution in the studied reaction is about 2 to 5 times higher than that of the undoped catalysts and it depends on palladium precursor. It is worth emphasizing that the activity of palladium catalysts prepared using palladium nitrate precursor was higher than that of palladium catalysts obtained using PdCl2 precursor at 290 °C. Those results showed that the activity of ternary and palladium catalysts for methanol synthesis depends on the composition of the catalysts, which points to a role of the oxide matrix on the catalytic properties.
The catalytic activity results of the ternary catalysts in the methanol synthesis reaction show that a decrease of copper loading leads to an enhanced of methanol yield. Ternary oxide catalysts showed comparable catalytic activities at both temperatures. However, the activity measurements clearly showed that the highest methanol production was observed for 0.25CuO:0.5Cr2O3:Al2O3 system.
Another tendency was observed for palladium catalysts prepared by the impregnation method using palladium chloride precursor. In this case, the 5 %Pd/0.25CuO:0.5Cr2O3:Al2O3 catalyst showed the highest yield in methanol synthesis, which contain the lowest loading of CuO oxide.
On the other hand, the catalytic systems impregnated by palladium nitrate precursor exhibited the value of catalytic activity on the similar level. At 260 °C, the yield of methanol formation is in the range 11–18 gMetOH/kgcat h, while at 290 °C, the yield is equal from 41 to 49 gMetOH/kgcat h. At the temperature 260 °C, the most promising catalysts were 5 %Pd/CuO:0.5Cr2O3:Al2O3 and 5 %Pd/0.5CuO:0.5Cr2O3:Al2O3 (18 gMetOH/kgcat h), which had similar specific surface area. The highest yield in the methanol synthesis reaction at 290 °C was achieved in the 5 %Pd/0.25CuO:0.5Cr2O3:Al2O3 and 5 %Pd/CuO:2Cr2O3:Al2O3 systems. The activity measurements showed that a simple correlation could not be found between the catalysts composition and the yield of methanol formation for all studied systems.
Wang and Lu [25] studied the physicochemical and catalytic properties of bimetallic Pd–Cu supported catalysts in CO oxidation. The catalysts were synthesized from different precursors. The characterization results confirmed that catalysts prepared using chloride and sulfate precursors lead to the formation of highly dispersed Cu metallic particles, while the use of acetate and nitrate precursors lead to the formation of large aggregated Cu particles. The authors also reported that highly dispersed catalysts exhibited high activity in CO oxidation. For bimetallic palladium catalysts prepared by co-impregnation using chloride and sulfate as a precursors, alloy formation between Pd and Cu was proved. The alloy formation can be the reason for the excellent catalytic properties for CO oxidation.
The hydrogenation of CO2 over bimetallic Pd–Cu catalysts was studied by Melián-Cabrera et. al. [26]. The authors claimed that incorporation of Pd to Cu–ZnO(Al2O3) catalysts enhance the CO2 conversion. The increase of methanol production authors explained by spillover effect between CuO and Pd. Hydrogen split from metallic palladium to CuO what facilitates the reduction of copper(II) oxide. The spillover would balance the oxidizing effect of water, and/or CO2, at the Cu surface.
Activity measurements obtained from methanol synthesis carried out over ternary and palladium ternary catalysts and reduction studies confirmed that palladium addition improves the yield in methanol production and facilitates the reduction of the oxide forms of ternary systems. This promotional effect can be explained by spillover effect between Pd and the oxide form of ternary systems (xCuO:yCr2O3:zAl2O3).
Conclusions
The activity result showed that the most promising systems are palladium supported catalysts Pd/Cu–Cr–Al synthesized by the impregnation method using palladium nitrate solution as a precursor. The most active system has proven to be palladium catalyst containing ternary system with following molar ratio 0.25CuO:0.5Cr2O3:Al2O3. The increase of efficiency of palladium catalysts in the methanol synthesis is caused by spillover effect. The reduction behavior of ternary oxide and palladium ternary oxide catalysts depends on the composition of the catalytic system. It is worth noting that palladium addition facilitates the reduction of oxide species presence on catalysts surface. The formation of spinel structures CuAl2O4 and CuCr2O4 and the crystalline phase Al2Cu2Cr2O8 were confirmed during calcination step by XRD.
References
Santiago M, Barbera K, Ferreira C, Curulla-Ferré C, Kolb P, Pérez-Ramírez J (2012) Catal Commun 21:63–67
Yaqing C, Xiaonian L, Huazhang L (2006) Chin J Catal 27(3):210–216
Hu B, Fujimoto K (2008) Appl Catal A 346:174–178
Cheng WH (1999) Acc Chem Res 32:685–691
Harolda MP, Nair B, Kolios G (2003) Chem Eng Sci 58:2551–2571
Cheekatamarla PK, Finnerty CM (2006) J Power Sources 160:490–499
Tsubaki N, Ito M (2001) Fujimoto K 197:224–227
Mierczynski P, Maniecki TP, Chalupka K, Maniukiewicz W, Jozwiak WK (2011) Catal Today 176:21–27
Ma L, Wainwright MS (1999) Appl Catal A 187:89–98
Ohyama S (2003) Top Catal 22:337–343
Kugai J, Miller JT, Guo N, Song C (2011) J Catal 277:46–53
Chang CC, Hsu CC, Chang CT, Chen YP, Liaw BJ, Chen YZ (2012) Int J Hydrogen Energy 37:11176–11184
Maniecki TP, Mierczynski P, Maniukiewicz W, Bawolak K, Gebauer D, Jozwiak WK (2009) Catal Lett 130:481–488
Mierczynski P, Maniecki TP, Maniukiewicz W, Jozwiak WK (2011) Reac Kinet Mech Cat 104:139–148
Słoczyński J, Grabowski R, Kozłowska A, Olszewski P, Stoch J, Skrzypek J, Lachowska M (2004) Appl Catal A 278:11–23
Epling WS, Hoflund GB, Minahan DM (1999) Appl Catal A 183:335–343
Luo M-F, Fang P, He M, Xie Y-L (2005) J Mol Catal A 239:243–248
Fand SN, Lin PY, Fu YL (1994) J Mol Catal 8:86
Amin NAS, Tan EF, Manan ZA (2004) J Catal 222:100–106
Abu-Zied BM, El-Awad AM (2001) J Mol Catal A 176:227–246
Maniecki TP, Mierczynski P, Maniukiewicz W, Jozwiak W (2011) Kinet Catal 52(6):835–842
Mierczynski P, Vasilev K, Mierczynska A, Maniukiewicz W, Maniecki TP (2013) Topics in Catalysis (in press)
Cubeiro ML, Fierro JLG (1998) J Catal 179:150–162
Mierczynski P, Maniukiewicz W, Maniecki TP (2013) Cent Eur J Chem (in press)
Wang F, Lu G (2010) Int J Hydrogen Energy 35:7253–7260
Melian-Cabrera I, Lopez Granados M, Terreros P, Fierro JLG (1998) Catal Today 45:251–256
Acknowledgments
This publication was prepared under scholarship START Programme funded by the Foundation of Polish Science. Partially financed from grant number 0680/B/H03/2011/40 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mierczynski, P., Kaczorowski, P., Maniecki, T.P. et al. The influence of Pd loading on the physicochemical properties of the Cu–Cr–Al methanol synthesis catalysts. Reac Kinet Mech Cat 109, 13–27 (2013). https://doi.org/10.1007/s11144-013-0550-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0550-9