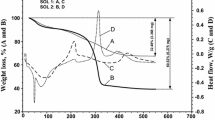
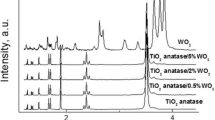
Abstract
Nanocomposite thin films of TiO2/WO3 are prepared by the sol–gel method on glass substrates using a dip-coating technique. The initial precursors of composite sols are chloride salts of the respective metals dissolved in ethanol with the addition of hydrogen peroxide as a catalyst of the hydrolysis and sol stabilization. The WO3 content in the films is selected to be 5 or 10%. The film thickness is controlled by the number of coating cycles. The films are dried between the successive coatings and finally annealed at 520 °C in air for 2 h. The morphology and phase composition of the composite films is characterized by SEM/EDX and X-ray analysis. The photocatalytic action of the films is tested with respect to the degradation of malachite green in water solutions under UV and visible light irradiation. The composite films TiO2/WO3 with 10% of WO3 always exhibit a better photocatalytic activity than the pure TiO2 films even under strong visible light.









Similar content being viewed by others
References
Fujishima A, Zhang X, Truck D (2007) Int J Hydrogen Energy 32:2664
Rehman S, Ullah R, Butt AM, Gohar ND (2009) J Hazard Mater 170:560
Chatterjee D, Dasgupta S (2005) J Photochem Photobiol C 6:186
Fujishima A, Zhang X, Tryk DA (2008) Surface Sci Rep 63:515
Serpone N, Borgarello E, Gratzel M (1984) J Chem Soc Chem Commun 6:342
Lo SC, Lin CF, Wu CH, Hsieh PH (2004) J Hazard Mater 114:183
Ho W, Yu JC (2006) J Mol Catal A 247:268
Bessekhouad Y, Robert D, Weber JV (2004) J Photochem Photobiol A 163:569
Wu L, Yu JC, Fua X (2006) J Mol Catal A 244:25
Zorn ME, Tompkins DT, Zeltner WA, Anderson MA (1999) Appl Catal B 23:1
Hernández-Alonso MD, Tejedor-Tejedor I, Coronado JM, Soria J, Anderson MA (2006) Thin Solid Films 502:125
Jianhua L, Rong Y, Songmei L (2006) Rare Met 25:636
Stoyanov S, Mladenova D, Dushkin C (2006) React Kinet Catal Lett 88:277
Rampaul A, Parkin IP, O’Neill SA, DeSouza J, Mills A, Elliott N (2003) Polyhedron 22:35
Puddu V, Mokaya R, Li Puma G (2007) Chem Commun 1
Bojinova AS, Papazova CI, Karadjova IB, Poulios I (2008) Eurasian J Anal Chem 2:34
Kubacka A, Fernández-García M, Colón G (2008) J Catal 254:272
Lorret O, Francova D, Waldner G, Stelzer N (2009) Appl Catal B 91:39
Srivastava S, Sinha R, Roy D (2004) Aquat Toxicol 66:319
Bergwerff A, Scherpenisse P (2003) J Chromatogr B 788:351
Culp S, Blankenship L, Kussewitt D, Doerge D, Mulligan L, Beland F (1999) Cemico-Biol Interact 122:153
Safaric I, Safarikova M (2002) Water Res 36:196
Bojinova A, Dushkin C, Eliyas A, Stoyanova-Eliyas E (2010) SIZEMAT2—September 19–21. Nessebar, Bulgaria, FOP13
Bojinova A, Dushkin C (2009) In: Balabanova E, Dragieva I (eds) Nanoscience & nanotechnology, vol 9, Heron Press, Sofia, p 84
Pecquenard B, Lecacheux H, Livage J, Julien C (1998) J Solid State Chem 135:159
Muhlebach J, Muller K, Schwarzenbach G (1970) Inorg Chem 9:2381
Dushkin C, Stoyanov S, Bojinova A, Russev S (2006) Ann Univ Sof Fac Chim 98–99:73
Bojinova A, Kralchevska R, Poulios I, Dushkin C (2007) Mater Chem Phys 106:187
Kostadinov M, Georgiev P, Bojinova A, Dushkin C (2003) In: Balabanova E, Dragieva I (eds) Nanoscience and nanotechnology, vol 3, Heron Press, Sofia, p 207
Choi W, Ko JY, Park H, Chung JS (2001) Appl Catal B 31:209
Kajitvichyanukul P, Ananpattarachai J, Pongpom S (2005) Sci Technol Adv Mater 6:352
Církva V, Žabová H, Hájek M (2008) J Photochem Photobiol A 198:13
Quici N, Vera ML, Choi H, Puma GL, Dionysiou D, Litter M, Destaillats H (2010) Appl Catal B 95:312
Acknowledgments
This research is financially supported by the project NATO SfP 982835 and DO 02-252 of the National Science Fund of Bulgaria (NSFB). C.D. is thankful also to COST D-43 Action of EC and project DO 02-82 UNION of NSFB.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bojinova, A., Dushkin, C. Photodegradation of malachite green in water solutions by means of thin films of TiO2/WO3 under visible light. Reac Kinet Mech Cat 103, 239–250 (2011). https://doi.org/10.1007/s11144-011-0295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0295-2