Abstract
Background
Patient-reported outcome measures (PROMs) could play an important role in identifying patients’ needs and goals in clinical encounters, improving communication and decision-making with clinicians, while making care more patient-centred. Comprehensive evidence that PROMS are an effective intervention is lacking in single randomised controlled trials (RCTs).
Methods
A systematic search was performed using controlled vocabulary related to the terms: clinical care setting and patient-reported outcome. English language studies were included if they were a RCT with a PROM as an intervention in a patient population. Included studies were analysed and their methodologic quality was appraised using the Cochrane Risk of Bias tool. The protocol was registered with PROSPERO (CRD42016034182).
Results
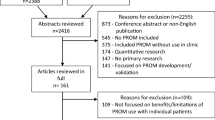
Of 4302 articles initially identified, 115 underwent full-text review resulting in 22 studies reporting on 25 comparisons. The majority of included studies were conducted in USA (11), among cancer patients (11), with adult participants only (20). Statistically significant and robust improvements were reported in the pre-specified outcomes of the process of care (2) and health care (3). Additionally, five, eight and three statistically significant but possibly non-robust findings were reported in the process of care, health and patient satisfaction outcomes, respectively.
Conclusions
Overall, studies that compared PROM to standard care either reported a positive effect or were not powered to find pre-specified differences. There is justification for the use of a PROM as part of standard care, but further adequately powered studies on their use in different contexts are necessary for a more comprehensive evidence base.

Similar content being viewed by others
Abbreviations
- ± PROM studies:
-
Studies that compared patient completion of a PROM with standard care in the control group
- PROM ± summary studies:
-
Studies in which all patients completed a PROM and compared the presentation of PROM summary scores to clinicians vs. no presentation of summary scores
- FDA:
-
Food and drug administration
- HRQL:
-
Health-related quality of life
- PRO:
-
Patient-reported outcomes
- PROM:
-
Patient-reported outcome measure
- QOL:
-
Quality of life
- RCT:
-
Randomised controlled trial
- SR:
-
Systematic review
References
Mayo, E. N., et al. (2015). ISOQOL dictionary of quality of life and health outcomes measurement. First Edition.
Varadhan, R., Segal, J. B., Boyd, C. M., Wu, A. W., & Weiss, C. O. (2013). A framework for the analysis of heterogeneity of treatment effect in patient-centered outcomes research. Journal of Clinical Epidemiology, 66, 818–825.
Greenhalgh, J., & (Feb (2009). The applications of PROs in clinical practice: What are they, do they work, and why? Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation, 18(1), 115–123.
Ahmed, S., Berzon, R. A., Revicki, D. A., Lenderking, W. R., Moinpour, C. M., Basch, E., et al. (2012). The use of patient-reported outcomes (PRO) within comparative effectiveness research: Implications for clinical practice and health care policy. Medical Care, 50(12), 1060–1070.
Wu, A. W., Snyder, C., Clancy, C. M., & Steinwachs, D. M. (2010). Adding the patient perspective to comparative effectiveness research. Health Affairs, 29(10), 1863–1871.
Antunes, B., Harding, R., Irene, J. H., & on behalf EUROIMPACT (2014). Implementing patient-reported outcome measures in palliative care clinical practice: A systematic review of facilitators and barriers. Palliative Medicine, 28(2), 158–175.
Burns, P. B., Rohrich, R. J., & Chung, K. C. (2011). The levels of evidence and their role in evidence-based medicine. Plastic and Reconstructive Surgery, 128(1), 305–310. https://doi.org/10.1097/PRS.0b013e318219c171.
Greenhalgh, J., & Meadows, K. (1999). The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: A literature review. Journal of Evaluation in Clinical Practice, 5(4), 401–416.
Espallargues, M., Valderas, J. M., & Alonso, J. (2000). Provision of feedback on perceived health status to health care professionals: A systematic review of its impact. Medical Care, 38(2), 175–186.
Gilbody, S. M., House, A. O., & Sheldon, T. (2002). Routine administration of health related quality of life (HRQoL) and needs assessment instruments to improve psychological outcome—A systemic review. Psychological Medicine, 32(8), 1345–1356.
Kotronoulas, G., Kearney, N., Maguire, R., Harrow, A., Di Domenico, D., Croy, S., et al. (2014). What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. Journal of Clinical Oncology, 32(14), 1480–1501.
Valderas, J. M., Kotzeva, A., Espallargues, M., Guyatt, G., Ferrans, C. E., Halyard, M. Y., et al. (2008). The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Quality of Life Research, 17(2), 179–193.
Marshall, S., Haywood, K., & Fitzpatrick, R. (2006). Impact of patient-reported outcome measures on routine practice: A structured review. Journal of Evaluation in Clinical Practice, 12(5), 559–568.
Alsaleh, K. (2013). Routine administration of standardized questionnaires that assess aspects of patients’ quality of life in medical oncology clinics: A systematic review. Journal of the Egyptian National Cancer Institute, 25(2), 63–70. https://doi.org/10.1016/j.jnci.2013.03.001.
Boyce, M. B., & Browne, J. P. (2013). Does providing feedback on patient-reported outcomes to healthcare professionals result in better outcomes for patients? A systematic review. Quality of Life Research, 22(9), 2265–2278. https://doi.org/10.1007/s11136-013-0390-0.
Chen, J., Ou, L., & Hollis, S. J. (2013). A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Services Research. https://doi.org/10.1186/1472-6963-13-211.
Greenhalgh, J., Dalkin, S., Gooding, K., Gibbons, E., Wright, J., Meads, D., et al. (2017). Functionality and feedback: A realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Health Services and Delivery Research, 5(2), 1–280.
Etkind, S. N., Daveson, B. A., Kwok, W., Witt, J., Bausewein, C., Higginson, I. J., et al. (2015). Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: Does it make a difference? A systematic review. Journal of Pain and Symptom Management, 49(3), 611–624. https://doi.org/10.1016/j.jpainsymman.2014.07.010.
Gutteling, J. J., Darlington, A. S., Janssen, H. L., Duivenvoorden, H. J., Busschbach, J. J. V., & De Man, R. A. (2008). Effectiveness of health-related quality-of-life measurement in clinical practice: A prospective, randomized controlled trial in patients with chronic liver disease and their physicians. Quality of Life Research, 17(2), 195–205. https://doi.org/10.1007/s11136-008-9308-7.
Lambert, M. J., Whipple, J. L., Hawkins, E. J., Vermeersch, D. A., Nielsen, S. L., & Smart, D. W. (2003). Is it time for clinicians to routinely track patient outcome? A meta-analysis. Clinical Psychology: Science and Practice, 10(3), 288–301. https://doi.org/10.1093/clipsy/bpg025.
Luckett, T., Butow, P. N., & King, M. T. (2009). Improving patient outcomes through the routine use of patient-reported data in cancer clinics: Future directions. Psycho-oncology, 18(11), 1129–1138.
Guyatt, G. H., Van Zanten, S. V., Feeny, D. H., & Patrick, D. L. (1989). Measuring quality of life in clinical trials: A taxonomy and review. CMAJ: Canadian Medical Association Journal, 140(12), 1441–1448.
Williams, K., Sansoni, J., Morris, D., Grootemaat, P., & Thompson, C. (2016). Patient-reported outcome measures: Literature review. Sydney: ACSQHC.
Boyce, M. B., Browne, J. P., & Greenhalgh, J. (2014). The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research. BMJ Quality and Safety, 23(6), 508–518. https://doi.org/10.1136/bmjqs-2013-002524.
Duncan, E. A. S., & Murray, J. (2012). The barriers and facilitators to routine outcome measurement by allied health professionals in practice: A systematic review. BMC Health Services Research. https://doi.org/10.1186/1472-6963-12-96.
Valderas, J. M., & Alonso, J. (2008). Patient reported outcome measures: A model-based classification system for research and clinical practice. Quality of Life Research, 17(9), 1125–1135. https://doi.org/10.1007/s11136-008-9396-4.
Schulz, K. F., Altman, D. G., & Moher, D. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology, 63(8), 834–840. https://doi.org/10.1016/j.jclinepi.2010.02.005.
Ishaque, S., Salter, A., Karnon, J., Chan, G., Nair, R. (2016). A systematic review of randomized clinical trials evaluating the use of patient reported outcome measures (PROMs) to improve patient outcomes. PROSPERO CRD42016034182. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016034182.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. https://doi.org/10.1186/2046-4053-4-1
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928.
Basch, E., Deal, A. M., Kris, M. G., Scher, H. I., Hudis, C. A., Sabbatini, P., et al. (2016). Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. Journal of Clinical Oncology, 34(6), 557–565.
Berry, D. L., Blumenstein, B. A., Halpenny, B., Wolpin, S., Fann, J. R., Austin-Seymour, M., et al. (2011). Enhancing patient-provider communication with the electronic self-report assessment for cancer: A randomized trial. Journal of Clinical Oncology, 29(8), 1029–1035.
Boyes, A., Newell, S., Girgis, A., McElduff, P., & Sanson-Fisher, R. (2006). Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? European Journal of Cancer Care, 15(2), 163–171.
Calkins, D. R., Rubenstein, L. V., Cleary, P. D., Davies, A. R., Jette, A. M., Fink, A., et al. (1994). Functional disability screening of ambulatory patients—A randomized controlled trial in a hospital-based group practice. Journal of General Internal Medicine, 9(10), 590–592.
Cleeland, C. S., Wang, X. S., Shi, Q., Mendoza, T. R., Wright, S. L., Berry, M. D., et al. (2011). Automated symptom alerts reduce postoperative symptom severity after cancer surgery: A randomized controlled clinical trial. Journal of Clinical Oncology, 29(8), 994–1000.
De Wit, M., Delemarre-van De Waal, H. A., Bokma, J. A., Haasnoot, K., Houdijk, M. C., Gemke, R. J., et al. (2008). Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: A randomized controlled trial. Diabetes Care, 31(8), 1521–1526.
Detmar, S. B., Muller, M. J., Schornagel, J. H., Wever, L. D. V., & Aaronson, N. K. (2002). Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. Journal of the American Medical Association, 288(23), 3027–3034.
Goldsmith, G., & Brodwick, M. (1989). Assessing the functional status of older patients with chronic illness. Family Medicine, 21(1), 38–41.
Hoekstra, J., de Vos, R., van Duijn, N. P., Schade, E., & Bindels, P. J. (2006). Using the symptom monitor in a randomized controlled trial: The effect on symptom prevalence and severity. Journal of Pain & Symptom Management, 31(1), 22–30.
Kazis, L. E., Callahan, L. F., Meenan, R. F., & Pincus, T. (1990). Health status reports in the care of patients with rheumatoid arthritis. Journal of Clinical Epidemiology, 43(11), 1243–1253.
McLachlan, S. A., Allenby, A., Matthews, J., Wirth, A., Kissane, D., Bishop, M., et al. (2001). Randomized trial of coordinated psychosocial interventions based on patient self-assessments versus standard care to improve the psychosocial functioning of patients with cancer. Journal of Clinical Oncology, 19(21), 4117–4125.
Mills, M. E., Murray, L. J., Johnston, B. T., Cardwell, C., & Donnelly, M. (2009). Does a patient-held quality-of-life diary benefit patients with inoperable lung cancer? Journal of Clinical Oncology, 27(1), 70–77.
Qureshi, N., Standen, P. J., Hapgood, R., & Hayes, J. (2001). A randomized controlled trial to assess the psychological impact of a family history screening questionnaire in general practice. Family Practice, 18(1), 78–83.
Rosenbloom, S. K., Victorson, D. E., Hahn, E. A., Peterman, A. H., & Cella, D. (2007). Assessment is not enough: A randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psycho-oncology, 16(12), 1069–1079.
Rubenstein, L. V., Calkins, D. R., Young, R. T., Cleary, P. D., Fink, A., Kosecoff, J., et al. (1989). Improving patient function: A randomized trial of functional disability screening. Annals of Internal Medicine, 111(10), 836–842.
Rubenstein, L. V., McCoy, J. M., Cope, D. W., Barrett, P. A., Hirsch, S. H., Messer, K. S., et al. (1995). Improving patient quality of life with feedback to physicians about functional status. Journal of General Internal Medicine, 10(11), 607–614.
Ruland, C. M., Holte, H. H., Røislien, J., Heaven, C., Hamilton, G. A., Kristiansen, J., et al. (2010). Effects of a computer-supported interactive tailored patient assessment tool on patient care, symptom distress, and patients’ need for symptom management support: A randomized clinical trial. Journal of the American Medical Informatics Association, 17(4), 403–410.
Street, R. L., Gold, W. R., & McDowell, T. (1994). Using health status surveys in medical consultations. Medical Care, 32(7), 732–744.
Velikova, G., Booth, L., Smith, A. B., Brown, P. M., Lynch, P., Brown, J. M., et al. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. Journal of Clinical Oncology, 22(4), 714–724.
Wagner, A. K., Ehrenberg, B. L., Tran, T. A., Bungay, K. M., Cynn, D. J., & Rogers, W. H. (1997). Patient-based health status measurement in clinical practice: A study of its impact on epilepsy patients’ care. Quality of Life Research, 6(4), 329–341.
Wasson, J., Hays, R., Rubenstein, L., Nelson, E., Leaning, J., Johnson, D., et al. (1992). The short-term effect of patient health status assessment in a health maintenance organization. Quality of Life Research, 1(2), 99–106.
Wolfe, J., Orellana, L., Cook, E. F., Ullrich, C., Kang, T., Geyer, J. R., et al. (2014). Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: Results from the PediQUEST randomized controlled trial. Journal of Clinical Oncology, 32(11), 1119–1126.
Measuring Impact—SF-36. (2018). Accessed September 2, 2018, from http://www.measuringimpact.org/s4-sf-36.
Skelly, A. C. (2011). Probability, proof, and clinical significance. Evidence-Based Spine-Care Journal, 2(4), 9–11.
Hays, R. D., & Woolley, J. M. (2000). The concept of clinically meaningful difference in health-related quality-of-life research: How meaningful is it? PharmacoEconomics, 18(5), 419–423.
El Gaafary, M. (2016). A guide to PROMs methodology and selection criteria. In Y. El Miedany (Ed.), Patient reported outcome measures in rheumatic diseases (pp. 21–58). Cham: Springer.
Cappelleri, J. C., & Bushmakin, A. G. (2014). Interpretation of patient-reported outcomes. Statistical Methods in Medical Research, 23(5), 460–483. https://doi.org/10.1177/0962280213476377.
Coon, C. D., & Cappelleri, J. C. (2016). Interpreting change in scores on patient-reported outcome instruments. Therapeutic Innovation and Regulatory Science, 50(1), 22–29. https://doi.org/10.1177/2168479015622667.
Dreyer, R. P., Jones, P. G., Kutty, S., et al. (2016). Quantifying clinical change: Discrepencies between patient’s and providers’ perspectives. Quality of Life Research, 25(9), 2213–2220.
Hemmingsson, H., Ólafsdóttir, L. B., & Egilson, S. T. (2017). Agreements and disagreements between children and their parents in health-related assessments. Disability and Rehabilitation, 39(11), 1059–1072.
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ: British Medical Journal. https://doi.org/10.1136/bmj.g7647.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This review does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed Consent was not applicable to this review as no primary data were collected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ishaque, S., Karnon, J., Chen, G. et al. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs). Qual Life Res 28, 567–592 (2019). https://doi.org/10.1007/s11136-018-2016-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-2016-z