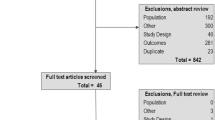
Abstract
Purpose
Patient preference information (PPI) have an increasing role in regulatory decision-making, especially in benefit–risk assessment. PPI can also facilitate prioritization of symptoms to treat and inform meaningful selection of clinical trial endpoints. We engaged patients and caregivers to prioritize symptoms of Duchenne and Becker muscular dystrophy (DBMD) and explored preference heterogeneity.
Methods
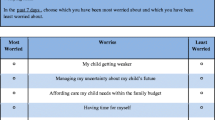
Best–worst scaling (object case) was used to assess priorities across 11 symptoms of DBMD that impact quality of life and for which there is unmet need. Respondents selected the most and least important symptoms to treat among a subset of five. Relative importance scores were estimated for each symptom, and preference heterogeneity was identified using mixed logit and latent class analysis.
Results
Respondents included patients (n = 59) and caregivers (n = 96) affected by DBMD. Results indicated that respondents prioritized “weaker heart pumping” [score = 5.13; 95% CI (4.67, 5.59)] and pulmonary symptoms: “lung infections” [3.15; (2.80, 3.50)] and “weaker ability to cough” [2.65; (2.33, 2.97)] as the most important symptoms to treat and “poor attention span” as the least important symptom to treat [− 5.23; (− 5.93, − 4.54)]. Statistically significant preference heterogeneity existed (p value < 0.001). At least two classes existed with different priorities. Priorities of the majority latent class (80%) reflected the aggregate results, whereas the minority latent class (20%) did not distinguish among pulmonary and other symptoms.
Conclusions
Estimates of the relative importance for symptoms of Duchenne muscular dystrophy indicated that symptoms with direct links to morbidity and mortality were prioritized above other non-skeletal muscle symptoms. Findings suggested the existence of preference heterogeneity for symptoms, which may be related to symptom experience.


Similar content being viewed by others
Change history
18 November 2022
ORCID of first author is updated.
References
Emery, A. E. (2002). The muscular dystrophies. The Lancet, 359(9307), 687–695.
Emery, A. E. (1991). Population frequencies of inherited neuromuscular diseases: A world survey. Neuromuscular Disorders, 1(1), 19–29.
Mah, J. K., Korngut, L., Dykeman, J., Day, L., Pringsheim, T., & Jette, N. (2014). A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscular Disorders, 24(6), 482–491.
Bushby, K., Finkel, R., Birnkrant, D. J., Case, L. E., Clemens, P. R., Cripe, L., et al. (2010). Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. The Lancet. Neurology, 9(1), 77–93.
Cyrulnik, S. E., Fee, R. J., Batchelder, A., Kiefel, J., Goldstein, E., & Hinton, V. J. (2008). Cognitive and adaptive deficits in young children with Duchenne muscular dystrophy (DMD). Journal of the International Neuropsychological Society, 14(5), 853–861.
Guglieri, M., Bushby, K., McDermott, M. P., Hart, K. A., Tawil, R., & Martens, W. B. (2017). Developing standardized corticosteroid treatment for Duchenne muscular dystrophy. Contemporary Clinical Trials, 58, 34–39.
Traynor, K. (2017). Deflazacort approved for Duchenne muscular dystrophy. American Journal of Health-System Pharmacy, 74(6), 368.
Aartsma-Rus, A., & Krieg, A. M. (2017). FDA approves Eteplirsen for Duchenne muscular dystrophy: The next chapter in the Eteplirsen saga. Nucleic Acid Therapeutics, 27(1), 1–3.
Unger, E. F., & Califf, R. M. (2017). Regarding “Eteplirsen for the treatment of Duchenne muscular dystrophy”. Annals of Neurology, 81(1), 162–164.
Niks, E. H., & Aartsma-Rus, A. (2017). Exon skipping: A first in class strategy for Duchenne muscular dystrophy. Expert Opinion on Biological Therapy, 17(2), 225–236.
Medical Device Innovation Consortium (MDIC). (2015). Patient centered benefit-risk project report: A framework for incorporating information on patient preferences regarding benefit and risk into regulatory assessments of new medical technology. Arlington: Medical Device Innovation Consortium (MDIC). Retrieved November 3, 2017, from http://mdic.org/wp-content/uploads/2015/05/MDIC_PCBR_Framework_Proof5_Web.pdf.
US Food and Drug Administration. (2016) Patient preference information—voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling: Guidance for industry, food and drug administration staff, and other stakeholders. Silver Spring: Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Food and Drug Administration. Retrieved November 3, 2017, from https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm446680.pdf.
Woodward, A. T. (2013). A latent class analysis of age differences in choosing service providers to treat mental and substance use disorders. Psychiatric Services, 64(11), 1087–1094.
Wong, Y. N., Egleston, B. L., Sachdeva, K., Eghan, N., Pirollo, M., Stump, T. K., et al. (2013). Cancer patients’ trade-offs among efficacy, toxicity and out-of-pocket cost in the curative and noncurative setting. Medical Care, 51(9), 838–845.
Whitty, J. A., Stewart, S., Carrington, M. J., Calderone, A., Marwick, T., Horowitz, J. D., et al. (2013). Patient preferences and willingness-to-pay for a home or clinic based program of chronic heart failure management: Findings from the which? trial. PLoS ONE, 8(3), e58347.
Waschbusch, D. A., Cunningham, C. E., Pelham, W. E. Jr., Rimas, H. L., Greiner, A. R., Gnagy, E. M., et al. (2011). A discrete choice conjoint experiment to evaluate parent preferences for treatment of young, medication naive children with ADHD. Journal of Clinical Child and Adolescent Psychology, 40(4), 546–561.
Naik-Panvelkar, M. P., Armour, C., Rose, J.,M., & Saini, B. (2012). Patient preferences for community pharmacy asthma services. PharmacoEconomics, 30(10), 961–976.
Lagarde, M. (2013). Investigating attribute non-attendance and its consequences in choice experiments with latent class models. Health Economics, 22(5), 554–567.
Guo, N., Marra, C. A., FitzGerald, J. M., Elwood, R. K., Anis, A. H., & Marra, F. (2011). Patient preference for latent tuberculosis infection preventive treatment: A discrete choice experiment. Value in Health, 14(6), 937–943.
Goossens, L. M., Utens, C. M., Smeenk, F. W., Donkers, B., van Schayck, O. C., & Rutten-van Mölken, M. P. (2014). Should I stay or should I go home? A latent class analysis of a discrete choice experiment on hospital-at-home. Value in Health, 17(5), 588–596.
Fraenkel, L., Suter, L., Cunningham, C. E., & Hawker, G. (2014). Understanding preferences for disease-modifying drugs in osteoarthritis. Arthritis Care and Research, 66(8), 1186–1192.
Cunningham, C. E., Chen, Y., Deal, K., Rimas, H., McGrath, P., Reid, G., et al. (2013). The interim service preferences of parents waiting for children’s mental health treatment: A discrete choice conjoint experiment. Journal of Abnormal Child Psychology, 41(6), 865–877.
Carroll, F. E., Al-Janabi, H., Flynn, T., & Montgomery, A. A. (2013). Women and their partners’ preferences for Down’s syndrome screening tests: A discrete choice experiment. Prenatal Diagnosis, 33(5), 449–456.
Brown, D. S., Poulos, C., Johnson, F. R., Chamiec-Case, L., & Messonnier, M. L. (2014). Adolescent girls’ preferences for HPV vaccines: A discrete choice experiment. Advances in Health Economics and Health Services Research, 24, 93–121.
Yan, K., Bridges, J. F., Augustin, S., Laine, L., Garcia-Tsao, G., & Fraenkel, L. (2015). Factors impacting physicians decisions to prevent variceal hemorrhage. BMC Gastroenterology, 15, 55.
Fraenkel, L., Lim, J., Garcia-Tsao, G., Reyna, V., Monto, A., & Bridges, J. F. P. (2016). Variation in treatment priorities for chronic Hepatitis C: A latent class analysis. The Patient, 9(3), 241–249.
US Food and Drug Administration. (2012) Guidance for industry and food and drug administration staff: Factors to consider when making benefit-risk determinations in medical device premarket approval and de novo classifications. Silver Spring: Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Food and Drug Administration. Retrieved November 3, 2017, from https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm517504.pdf.
Hunter, N. L., O’Callaghan, K. M., & Califf, R. M. (2015). Engaging patients across the spectrum of medical product development: View from the US Food and Drug Administration. Journal of the American Medical Association, 314(23), 2499–2500.
Ho, M. P., Gonzalez, J. M., Lerner, H. P., Neuland, C. Y., Whang, J. M., McMurry-Heath, M., et al. (2015). Incorporating patient-preference evidence into regulatory decision making. Surgical Endoscopy, 29(10), 2984–2993.
Hauber, B. A., Fairchild, A. O., & Johnson, R. F. (2013). Quantifying benefit-risk preferences for medical interventions: An overview of a growing empirical literature. Applied Health Economics and Health Policy, 11(4), 319–329.
van Til, J. A., & Ijzerman, M. J. (2014). Why should regulators consider using patient preferences in benefit-risk assessment? PharmacoEconomics, 32(1), 1–4.
US Food and Drug Administration. (2018) Duchenne muscular dystrophy and related dystrophinopathies: Developing drugs for treatment Guidance for industry. Silver Spring: Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Food and Drug Administration. Retrieved March 17, 2018, from https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM450229.pdf.
McNeil, D. E., Davis, C., Jillapalli, D., Targum, S., Durmowicz, A., & Coté, T. R. (2010). Duchenne muscular dystrophy: Drug development and regulatory considerations. Muscle and Nerve, 41(6), 740–745.
Peay, H. L., Hollin, I. L., Fischer, R., & Bridges, J. F. P. (2014). A community-engaged approach to quantifying caregiver preferences for the benefits and risks of emerging therapies for Duchenne muscular dystrophy. Clinical Therapeutics, 36(5), 624–637.
Peay, H. L., Sheffer, H., & Tibben, A. (2013). Expectations and decision making in clinical trials for Duchenne and Becker muscular dystrophy. In 18th international congress of the world muscle society, Asilomar.
Finn, A., & Louviere, J. J. (1992). Determining the appropriate response to evidence of public concern: The case of food safety. Journal of Public Policy and Marketing, 11(2), 12–25.
Marley, A. A., & Louviere, J. J. (2005). Some probabilistic models of best, worst, and best-worst choices. Journal of Mathematical Psychology, 49(6), 464–480.
Flynn, T. N. (2010). Valuing citizen and patient preferences in health: Recent developments in three types of best-worst scaling. Expert Review of Pharmacoeconomics and Outcomes Research, 10(3), 259–267.
Mühlbacher, A. C., Kaczynski, A., Zweifel, P., & Johnson, F. R. (2015). Experimental measurement of preferences in health and healthcare using best-worst scaling: An overview. Health Economics Review, 6(1), 1–14.
Flynn, T. N., Louviere, J. J., Peters, T. J., & Coast, J. (2007). Best-worst scaling: What it can do for health care research and how to do it. Journal of Health Economics, 26(1), 171–189.
Flynn, T. N., & Marley, A. (2014). Best-worst scaling: Theory and methods. In S. Hess & A. Daly (Eds.), Handbook of choice modelling (pp. 178–201). Cheltenham: Edward Elgar Publishing Limited.
Hollin, I. L., Young, C., Hanson, C., Bridges, J., & Peay, H. (2016). Developing a patient-centered benefit-risk survey: A community-engaged process. Value in Health, 19, 751–757.
Kuhfeld, W. (2010). Orthogonal arrays [TS-723]. Cary, NC: SAS.
Youden, W. J. (1940). Experimental designs to increase accuracy of greenhouse studies. Contributions. Boyce Thompson Institute for Plant Research, 11, 219–228.
Youden, W. J. (1937). Use of incomplete block replications in estimating tobacco-mosaic virus. Contributions from Boyce Thompson Institute, 9(1), 41–48.
Hauber, A. B., González, J. M., Groothuis-Oudshoorn, C. G., Prior, T., Marshall, D. A., Cunningham, C. et al. (2016). Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value in Health, 19(4):300–315.
Deal, K. (2014). Segmenting patients and physicians using preferences from discrete choice experiments. The Patient, 7(1), 5–21.
Acknowledgements
The authors appreciate the time and commitment of all the community members serving on the leadership, stakeholder, and review committees. We are indebted to all of the caregivers and individuals with Duchenne and Becker muscular dystrophy who participated in the survey. The authors wish to thank Caroline Hanson and Caroline Young for their help in designing the instrument and programming the data collection tool.
Funding
This study was funded by Parent Project Muscular Dystrophy (PPMD) (Grant Number 01212). Hollin and Bridges received support from a Grant (#01212) from Parent Project Muscular Dystrophy (PPMD). Peay was an employee of PPMD at the time of this research. PPMD received funding for this project from Santhera Pharmaceuticals. Bridges and Janssen also received support from a Patient-Centered Outcomes Research Institute (PCORI) Methods Program Award (ME-1303-5946) and through the Johns Hopkins-FDA Center for Excellence in Regulatory Science and Innovation (CERSI) (1U01FD004977-01). Peay currently receives support from a Patient-Centered Outcomes Research Institute (PCORI) PCORnet program award (PPRN-1306-04640-Phase II). Hollin is currently an employee of the National Pharmaceutical Council, although was not at the time of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Hollin, I.L., Peay, H., Fischer, R. et al. Engaging patients and caregivers in prioritizing symptoms impacting quality of life for Duchenne and Becker muscular dystrophy. Qual Life Res 27, 2261–2273 (2018). https://doi.org/10.1007/s11136-018-1891-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-1891-7