Abstract
Purpose
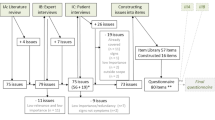
Despite the significant impact of Crohn’s disease (CD) on patients’ physical and emotional well-being, no CD-specific patient-reported outcome (PRO) measure is available for determining the efficacy of interventions. The objective of the study was to develop and validate the Crohn’s Life Impact Scale (CLIQ), the first such measure.
Methods
Questionnaire content was derived from qualitative interviews with CD patients and face and content validity assessed by cognitive debriefing interviews (CDIs) with patients. A postal survey was conducted to identify the final scale, confirm its unidimensionality and determine reproducibility and construct validity. A subset of the respondents was sent a second questionnaire package 2 weeks after the first. The survey included the CLIQ, Nottingham Health Profile (NHP) and Unidimensional Fatigue Impact Scale (U-FIS).
Results
Content analysis was conducted on the 30 interview transcripts and a draft scale produced. The CDIs indicated that the draft scale was relevant, clear and easy to use. The questionnaire package was completed by 273 CD patients (65.6 % male; aged 16–79 (mean 43.9; SD 15.1) years). Of these, 104 also completed the second package. Rasch analysis confirmed a 27-item unidimensional QoL scale (p < 0.05). Both internal consistency and test–retest reliability were high (0.91). Scores on the CLIQ were related to both physical and emotional impairments (NHP) and to fatigue (U-FIS).
Conclusion
The CLIQ, the first CD-specific PRO, is unidimensional and has excellent psychometric properties. It should prove to be a valuable tool for evaluating the impact of CD and its treatment from the patients’ perspective.




Similar content being viewed by others
References
Crohn’s and Colitis UK. Crohn’s Disease information booklet. http://www.crohnsandcolitis.org.uk/Resources/CrohnsAndColitisUK/Documents/Publications/Booklets/Crohns%20Disease.pdf. Accessed 24 July 2013.
Nahon, S., Lahmek, P., Saas, C., Durance, C., Olympie, A., Lesgourgues, B., & Gendre, J. P. (2011). Socioeconomic and psychological factors associated with nonadherence to treatment in inflammatory bowel disease patients: Results of the ISSEO survey. Inflammatory Bowel Diseases, 17(6), 1270–1276.
European Medicines Agency. (2006). Reflection paper on the regulatory guidance for the use of health-related quality of life measures in the evaluation of medicinal products. http://www.ema.europa.eu/ema/pages. Accessed September 3, 2013.
Fehnel, S., DeMuro, C., McLeod, L., Coon, C., & Gnanasakthy, A. (2013). US FDA patient-reported outcome guidance: Great expectations and unintended consequences. Expert Review of Pharmacoeconomics and Outcomes Research, 13(4), 441–446.
Abdovic, S., Mocic Pavic, A., Milosevic, M., Persic, M., Senecic-Cala, I., & Kolacek, S. (2013). The IMPACT-III (HR) questionnaire: a valid measure of health-related quality of life in Croatian children with inflammatory bowel disease. Journal of Crohn’s and Colitis, 7(11), 908–915.
Achleitner, U., Coenen, M., Colombel, J. F., Peyrin-Biroulet, L., Sahakyan, N., & Cieza, A. (2012). Identification of areas of functioning and disability addressed in inflammatory bowel disease-specific patient reported outcome measures. Journal of Crohn’s and Colitis, 6(5), 507–517.
Dür, M., Sadloňová, M., Haider, S., Binder, A., Stoffer, M., Coenen, M., et al. (2014). Health determining concepts important to people with Crohn’s disease and their coverage by patient-reported outcomes of health and wellbeing. Journal of Crohn’s and Colitis, 8(1), 45–55.
Huamán, J. W., Casellas, F., Borruel, N., Peláez, A., Torrejón, A., Castells, I., et al. (2010). Cutoff values of the Inflammatory Bowel Disease Questionnaire to predict a normal health related quality of life. Journal of Crohn’s and Colitis, 4(6), 637–641.
McDermott, E., Keegan, D., Byrne, K., Doherty, G. A., & Mulcahy, H. E. (2013). The Short Health Scale: A valid and reliable measure of health related quality of life in English speaking inflammatory bowel disease patients. Journal of Crohn’s and Colitis, 7(8), 616–621.
Werner, H., Landolt, M. A., Buehr, P., Koller, R., Nydegger, A., Spalinger, J., et al. (2014). Validation of the IMPACT-III quality of life questionnaire in Swiss children with inflammatory bowel disease. Journal of Crohn’s and Colitis, 8(7), 641–648.
Zijlstra, M., De Bie, C., Breij, L., van Pieterson, M., van Staa, A., de Ridder, L., et al. (2013). Self-efficacy in adolescents with inflammatory bowel disease: A pilot study of the “IBD-yourself”, a disease-specific questionnaire. Journal of Crohn’s and Colitis, 7(9), e375–e385.
Guyatt, G., Mitchell, A., Irvine, E. J., Singer, J., Williams, N., Goodacre, R., & Tompkins, C. (1989). A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology, 96(3), 804–810.
Ware, J. E, Jr, & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care, 30, 473–483.
van der Linden, W. J., & Hambleton, R. K. (Eds.). (1996). Handbook of modern item response theory. New York: Springer.
Irvine, E. J. (1999). Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality-of-life instrument for adult patients with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition, 28(4), S23–S27.
Bernklev, T., Moum, B., Moum, T., & Inflammatory Bowel South-Eastern Norway (IBSEN) Group of Gastroenterologists. (2002). Quality of life in patients with inflammatory bowel disease: Translation, data quality, scaling assumptions, validity, reliability and sensitivity to change of the Norwegian version of IBDQ. Scandinavian Journal of Gastroenterology, 37(10), 1164–1174.
Vlachonikolis, I. G., Pallis, A. G., & Mouzas, I. A. (2003). Improved validation of the Inflammatory Bowel Disease Questionnaire and development of a short form in Greek patients. The American Journal of Gastroenterology, 98, 1802–1812.
Watanabe, K., Funayama, Y., Fukushima, K., Shibata, C., Takahashi, K., Ogawa, H., et al. (2006). Assessment of the Japanese Inflammatory Bowel Disease Questionnaire in patients after ileal pouch anal anastomosis for ulcerative colitis. Journal of Gastroenterology, 41, 662–667.
Stjernman, H., Grännö, C., Bodemar, G., Järnerot, G., Ockander, L., Tysk, C., et al. (2006). Evaluation of the Inflammatory Bowel Disease Questionnaire in Swedish patients with Crohn’s disease. Scandinavian Journal of Gastroenterology, 41, 934–943.
Vidal, A., Gómez-Gil, E., Sans, M., Portella, M. J., Salamero, M., Piqué, J. M., & Panés, J. (2007). Psychometric properties of the original Inflammatory Bowel Disease Questionnaire, a Spanish version. Gastroenterología y Hepatología, 30, 212–218.
Devlen, J., Beusterien, K., Yen, L., Ahmed, A., Cheifetz, A. S., & Moss, A. C. (2014). The burden of inflammatory bowel disease: A patient-reported qualitative analysis and development of a conceptual model. Inflammatory Bowel Diseases, 20(3), 545–552.
Wright, B. D. (1996). Comparing Rasch measurement and factor analysis. Structural Equation Modelling, 3, 3–24.
Prieto, L., Alonso, J., & Lamarca, R. (2003). Classical test theory versus Rasch analysis for quality of life questionnaire reduction. Health and Quality of Life Outcomes, 1, 27.
Waugh, R. F., & Chapman, E. S. (2005). An analysis of dimensionality using factor analysis (true-score theory) and Rasch measurement: What is the difference? Which method is better? Journal of Applied Measurement, 6, 80–99.
Belvedere, S. L., & de Morton, N. A. (2010). Application of Rasch analysis in health care is increasing and is applied for variable reasons in mobility instruments. Journal of Clinical Epidemiology, 63(12), 1287–1297.
Siemons, L., Ten Klooster, P. M., Taal, E., Glas, C. A., & Van de Laar, M. A. (2012). Modern psychometrics applied in rheumatology—A systematic review. BMC Musculoskeletal Disorders, 13, 216.
Doward, L. C., Meads, D. M., & Thorsen, H. (2004). Requirements for Quality of Life instruments in clinical research. Value in Health, 7(s1), S13–S16.
Doyal, L., & Gough, I. (1991). A theory of human need. Basingstoke: Macmillan.
Hyde, M., Wiggins, R. D., Higgs, P., & Blane, D. B. (2003). A measure of quality of life in early old age: The theory, development and properties of a needs satisfaction model (CASP-19). Aging Mental Health, 7(3), 186–194.
Hornquist, J. O. (1982). The concept of quality of life. Scandinavian Journal of Social Medicine, 10(2), 57–61.
Doward, L. C., McKenna, S. P., Meads, D. M., Twiss, J., & Eckert, B. J. (2009). The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Multiple Sclerosis, 15(9), 1092–1102.
Hunt, S. M., & McKenna, S. P. (1992). The QLDS: A scale for the measurement of quality of life in depression. Health Policy, 22, 307–319.
Whalley, D., McKenna, S. P., de Jong, Z., & van der Heijde, D. (1997). Quality of life in rheumatoid arthritis. British Journal of Rheumatology, 36(8), 884–888.
Keenan, A. M., McKenna, S. P., Doward, L. C., Conaghan, P. G., Emery, P., & Tennant, A. (2008). Development and validation of a needs-based quality of life instrument for osteoarthritis. Arthritis and Rheumatism, 59(6), 841–848.
Doward, L. C., Spoorenberg, A., Cook, S. A., Whalley, D., Helliwell, P. S., Kay, L. J., et al. (2003). Development of the ASQoL: A quality of life instrument specific to ankylosing spondylitis. Annals of the Rheumatic Diseases, 62(1), 20–26.
McKenna, S. P., Doward, L. C., Whalley, D., Tennant, A., Emery, P., & Veale, D. J. (2004). Development of the PsAQoL: A quality of life instrument specific to psoriatic arthritis. Annals of the Rheumatic Diseases, 63(2), 162–169.
Linacre, J. M. (1994). Sample size and item calibration stability. Rasch Measurement Transactions, 7(4), 328.
Hunt, S. M., McEwen, J., & McKenna, S. P. (1986). Measuring health status. London: Croom Helm.
European Group for Quality of Life Assessment and Health Measurement. (1993). European guide to the Nottingham health profile. Brookwood: Brookwood Medical Publications.
Meads, D. M., Doward, L. C., McKenna, S. P., Fisk, J., Twiss, J., & Eckert, B. (2009). The development and validation of the Unidimensional Fatigue Impact Scale. Multiple Sclerosis, 15(10), 1228–1238.
van Langenberg, D. R., & Gibson, P. R. (2014). Factors associated with physical and cognitive fatigue in patients with Crohn’s disease: A cross-sectional and longitudinal study. Inflammatory Bowel Diseases, 20(1), 115–125.
van Langenberg, D. R., Della Gatta, P., Warmington, S. A., Kidgell, D. J., Gibson, P. R., & Russell, A. P. (2014). Objectively measured muscle fatigue in Crohn’s disease: correlation with self-reported fatigue and associated factors for clinical application. Journal of Crohn’s and Colitis, 8(2), 137–146.
Rasch, G. (1960). Probabilistic models for some intelligence and attainment tests. Chicago: University of Chicago Press.
Tennant, A., McKenna, S. P., & Hagell, P. (2004). Application of Rasch analysis in the development and application of quality of life instruments. Value in Health, 7(Suppl 1), S22–S26.
Andrich, D., Sheridan, B. S., & Luo, G. (2010). Rumm 2030: Rasch unidimensional measurement models [computer software]. Perth: RUMM Laboratory.
Holland, P. W., & Wainer, H. (Eds.). (1993). Differential item functioning. New Jersey: Lawrence Erlbaum Associates.
Tennant, A., & Gonaghan, P. G. (2007). The Rasch measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one should one look for in a Rasch paper? Arthritis and Rheumatism, 57, 1358–1362.
Balsamo, M., Giampaglia, G., & Saggino, A. (2014). Building a new Rasch-based self-report inventory of depression. Neuropsychiatric Disease and Treatment, 10, 153–165.
Smith, E. V. (2002). Detecting and evaluating the impact of multidimensionality using item fit statistics and principal component analysis of residuals. Journal of Applied Measurement, 3(2), 205–231.
Nunally, J. C, Jr. (1978). Psychometric theory (2nd ed.). New York: McGraw-Hill.
Weiner, E., & Stewart, B. (1984). Assessing individuals. Boston: Little Brown.
de Jong, Z., van der Heijde, D., McKenna, S. P., & Whalley, D. (1997). The reliability and construct validity of the RAQoL: A rheumatoid arthritis-specific quality of life instrument. British Journal of Rheumatology, 36(8), 878–883.
Kemp, K., Griffiths, J., & Lovell, K. (2012). Understanding the health and social care needs of people living with IBD: A meta-synthesis of the evidence. World Journal of Gastroenterology, 18(43), 6240–6249.
Kappelman, M. D., Long, M. D., Martin, C., DeWalt, D. A., Kinneer, P. M., Chen, W., et al. (2014). Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology, 12(8), 1315.
Kim, M., Han, H. R., & Phillips, L. (2003). Metric equivalence assessment in cross-cultural research: using an example of the Center for Epidemiological Studies-Depression Scale. Journal of Nursing Measurement, 11, 5–18.
Bowden, A., & Fox-Rushby, J. A. (2003). A systematic and critical review of the process of translation and adaptation of generic health-related quality of life measures in Africa, Asia, Eastern Europe, the Middle East, South America. Social Science and Medicine, 57, 1289–1306.
Acknowledgments
The authors would like to thank all the patients who contributed to the development and validation of the CLIQ. The study was part funded by Crohn’s and Colitis UK, and we would like to thank them for their support. The authors acknowledge the support of The National Institute for Health Research/Wellcome Trust Clinical Research Facility at Central Manchester University Hospitals NHS Foundation Trust.
Conflict of interest
None.
Ethical standards
The study procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, and its later amendments. Participants at each stage gave written informed consent prior to inclusion in the study. There is no identifying information in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilburn, J., McKenna, S.P., Twiss, J. et al. Assessing quality of life in Crohn’s disease: development and validation of the Crohn’s Life Impact Questionnaire (CLIQ). Qual Life Res 24, 2279–2288 (2015). https://doi.org/10.1007/s11136-015-0947-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-0947-1