Abstract
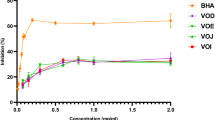
The MeOH:H2O (7:3) extracts of leaves from Chilean bean landraces were assessed for total phenolic (TP), total flavonoid (TF), total proanthocyanidin (TPA) content, antioxidant capacity (ORAC, FRAP, TEAC, CUPRAC, DPPH) and the inhibition of enzymes associated with metabolic syndrome (α-glucosidase, α-amylase, pancreatic lipase). The chemical profiles were analyzed by HPLC-DAD. Higher antioxidant activity in the ORAC and CUPRAC assay was found for the landrace Coscorrón, and the best effect in the TEAC for Sapito, respectively. The main phenolics were flavonol glycosides and caffeic acid derivatives. The extracts presented strong activity against α-glucosidase, but were inactive towards α-amylase and pancreatic lipase. The leaf extract from the Sapito landrace was fractionated to isolate the main α-glucosidase inhibitors, leading to caffeoylmalic acid with an IC50 of 0.21 μg/mL. The HPLC fingerprints of the leaves differentiate three groups of chemical profiles, according to the main phenolic content. A significant correlation was found between the α-glucosidase inhibition, the content of caffeoylmalic acid (r = −0.979) and kaempferol 3-O-β-D-glucoside (r = 0.942) in the extracts. The presence of α-glucosidase inhibitors in the leaves of Chilean beans support their potential as a source of bioactive compounds.
Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
Abbreviations
- CE:
-
Catechin equivalents
- CUPRAC:
-
Cupric reducing antioxidant capacity
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl radical
- FRAP:
-
Ferric reducing antioxidant power
- GAE:
-
Gallic acid equivalents
- HPLC:
-
High performance liquid chromatography
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- NPR:
-
Natural products reagent, diphenylboric acid-β-ethylamino ester
- ORAC:
-
Oxygen radical antioxidant capacity
- Rt :
-
Retention time in minutes
- SC50 :
-
Extract concentration scavenging 50% of the DPPH radical
- sh:
-
Shoulder
- TE:
-
Trolox equivalents
- TEAC:
-
Trolox equivalents antioxidant capacity
- TF:
-
Total flavonoid content
- TLC:
-
Thin layer chromatography
- TP:
-
Total phenolic content
- TPA:
-
Total proanthocyanidins
- UV:
-
Ultraviolet
References
Shamseldin A, Velázquez E (2020) The promiscuity of Phaseolus vulgaris L. (common bean) for nodulation with Rhizobia: a review. World J Microbiol Biotechnol 36:63. https://doi.org/10.1007/s11274-020-02839-w
Gentry HS (1969) Origin of the common beam, Phaseolus vulgaris. Economic Bot 23:55–69. https://www.jstor.org/stable/4253014. Accessed 4 Jan 2022
Paredes CM, Becerra VV, Tay UJ, Blair MW, Bascur BG (2010) Selection of a representative core collection from the Chilean common bean germplasm. Chilean J Agric Res 70:3–15. https://doi.org/10.4067/S0718-58392010000100001
Bai Y, Xu Y, Wang B, Li S, Guo F, Hua H, Zhao Y, Yu Z (2017) Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth periods leaves. J Funct Foods 35:694–702. https://doi.org/10.1016/j.jff.2017.06.036
Ghaly AE, Alkoaik FN (2010) Extraction of protein from common plant leaves for use as human food. Am J Appl Sci 7:331–342. https://doi.org/10.3844/ajassp.2010.331.342
Ha PTT, Tran NTB, Tram NTN, Kha VH (2020) Total phenolic, total flavonoid contents and antioxidant potential of common bean (Phaseolus vulgaris L.) in Vietnam. AIMS Agric Food 5:635–648. https://doi.org/10.3934/agrfood.2020.4.635
Yang Q-Q, Gan R-Y, Ge Y-Y, Zhang D, Corke H (2018) Polyphenols in common beans (Phaseolus vulgaris L.): chemistry, analysis, and factors affecting composition. Compr Rev Food Sci Food Saf 17:1518–1539. https://doi.org/10.1111/1541-4337.12391
Thompson HJ (2019) Dietary bean consumption and human health. Nutrients 17:3074. https://doi.org/10.3390/nu11123074
Moreno-Valdespino CA, Luna-Vital D, Camacho-Ruiz RM, Mojica L (2020) Bioactive proteins and phytochemicals from legumes mechanism of action preventing obesity and type-2 diabetes. Food Res Int 130:108905. https://doi.org/10.1016/j.foodres.2019.108905
Becerra-Tomás N, Papandreou C, Salas-Salvadó J (2019) Legume consumptions and cardiometabolic health. J Adv Nutr 10(Suppl_4):S437–S450. https://doi.org/10.1093/advances/nmz003
Aparicio-Fernández X, García-Gasca T, Yousef GG, Lila MA, González de Mejia E, Loarca-Pina G (2006) Chemopreventive activity of polyphenolics from black Jamapa bean (Phaseolus vulgaris L.) on HeLa and HaCaT cells. J Agric Food Chem 54:2116–2122. https://doi.org/10.1021/jf052974m
Frassinetti S, Gabriele M, Caltavuturo L, Longo V, Pucci L (2015) Antimutagenic and antioxidant activity of a selected lectin-free common bean (Phaseolus vulgaris L.) in two cell-based models. Plant Foods Hum Nutr 70:35–41. https://doi.org/10.1007/s11130-014-0453-6
García-Lafuente A, Moro C, Manchón N, Gonzalo-Ruiz A, Villares A, Guillamón E, Rostagno M, Mateo-Vivaracho L (2014) In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem 161:216–223. https://doi.org/10.1016/j.foodchem.2014.04.004
Gan R-Y, Deng Z-Q, Yan A-X, Shah NP, Lui W-Y, Chan C-L, Corke H (2016) Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. LWT-Food Sci Technol 73:168–177. https://doi.org/10.1016/j.lwt.2016.06.012
Damián-Medina K, Salinas-Moreno Y, Milenkovic D, Figueroa-Yáñez L, Marino-Marmolejo E, Higuera-Ciapara I, Vallejo-Cardona A, Lugo-Cervantes E (2020) In silico analysis of antidiabetic potential of phenolic compounds from blue corn (Zea mays L.) and black bean (Phaseolus vulgaris L.). Heliyon 6:e03632. https://doi.org/10.1016/j.heliyon.2020.e03632
Ramírez-Venegas G, De Ita-Pérez D, Díaz-Muñoz M, Méndez I, García-Gasca T, Ahumada-Solórzano M, Zambrano-Estrada X, Vázquez-Martínez O, Guzmán-Maldonado H, Luna-Moreno D (2021) Supplementation with Phaseolus vulgaris leaves improves metabolic alterations induced by high-fat/fructose diet in rats under time-restricted feeding. Plant Foods Hum Nutr 76:297–303. https://doi.org/10.1007/s11130-021-00904-9
Manousi N, Sarakatsianos I, Samanidou V (2019) 10 - Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. Grumezescu AM, Holban AM (eds) In: Engineering tools in the beverage industry. Woodhead Publishing, pp. 283–314. https://doi.org/10.1016/B978-0-12-815258-4.00010-X
Reyes-Martínez A, Almaraz-Abarca N, Gallardo-Velázquez T, González-Elizondo M del S, Herrera-Arrieta Y, Pajarito-Ravelero A, Alanís-Bañuelos RE, Torres-Morán MI (2014) Evaluation of foliar phenols of 25 Mexican varieties of common bean (Phaseolus vulgaris L.) as antioxidants and varietal markers. Nat Prod Res 28:2158–2162. https://doi.org/10.1080/14786419.2014.930855
Dinelli G, Bonetti A, Minelli M, Marotte I, Catizone P, Mazzanti A (2006) Content of flavonols in Italian bean (Phaseolus vulgaris L.) ecotypes. Food Chem 99:105–114. https://doi.org/10.1016/j.foodchem.2005.07.028
Šibul F, Orčić D, Vasić M, Anačkov G, Nađpal J, Savić A, Mimica-Dukić N (2016) Phenolic profile, antioxidant and anti-inflammatory potential of herb and root extracts of seven selected legumes. Ind Crop Prod 83:641–653. https://doi.org/10.1016/j.indcrop.2015.12.057
Söhretoglu D, Sari S (2020) Flavonoids as alpha-glucosidase inhibitors: mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem Rev 19:1081–1092. https://doi.org/10.1007/s11101-019-09610-6
Tadera K, Minami Y, Takamatsu K, Matsuoka T (2006) Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol 52:149–153. https://doi.org/10.3177/jnsv.52.149
Lim J, Ferruzzi MG, Hamaker BR (2022) Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem 370:130981. https://doi.org/10.1016/j.foodchem.2021.130981
Yoshihara T, Yoshikawa H, Kunimatsu S, Sakamura S, Sakuma T (1977) New amino acid derivatives conjugated with caffeic acid and DOPA from red clover (Trifolium pretense). Agric Biol Chem 41:1679–1684. https://doi.org/10.1080/00021369.1977.10862719
Szajwaj B, Moldoch J, Masullo M, Piacente S, Oleszek W, Stochmal A (2011) Amides and esters of phenylpropenoic acids from the aerial parts of Trifolium pallidum. Nat Prod Comm 6(9):1293–1296. https://doi.org/10.1177/1934578X1100600921
Grauso L, de Falco B, Lanzotti V, Motti R (2020) Stinging nettle, Urtica dioica L.: botanical, phytochemical and pharmacological overview. Phytochem Rev 19:1341–1377. https://doi.org/10.1007/s11101-020-09680-x
Sullivan ML, Green HA, Verdonk JC (2020) Engineering alfalfa to produce 2-O-caffeoyl-L-malate (phaselic acid) for preventing post-harvest protein loss via oxidation by polyphenol oxidase. Front Plant Sci 11:610399. https://doi.org/10.3389/fpls.2020.610399
Sullivan ML, Zeller WE (2013) Efficacy of various naturally occurring caffeic acid derivatives in preventing post-harvest protein losses in forages. J Sci Food Agric 93:219–226. https://doi.org/10.1002/jsfa.5781
Chen PX, Tang Y, Marcone MF, Pauls PK, Zhang B, Liu R, Tsao R (2015) Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem 185:298–308. https://doi.org/10.1016/j.foodchem.2015.03.100
Pitura K, Arntfield SD (2019) Characteristics of flavonol glycosides in bean (Phaseolus vulgaris L.) seed coats. Food Chem 272:26–32. https://doi.org/10.1016/j.foodchem.2018.07.220
Guajardo-Flores D, Serna-Saldívar SO, Gutiérrez-Uribe JA (2013) Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem 141:1497–1503. https://doi.org/10.1016/j.foodchem.2013.04.010
Funding
N.N. thanks CONICYT for a doctoral grant Folio 21192237. This research received funding from FONDECYT 1210076 and Proyecto Fortalecimiento al Desarrollo Científico de Centros Regionales-ANID R20F0001CEAP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 78 kb)
Rights and permissions
About this article
Cite this article
Alarcón-Espósito, J., Nina, N., Theoduloz, C. et al. Phenolic Composition and α-Glucosidase Inhibition of Leaves from Chilean Bean Landraces. Plant Foods Hum Nutr 77, 135–140 (2022). https://doi.org/10.1007/s11130-022-00955-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-022-00955-6