Abstract
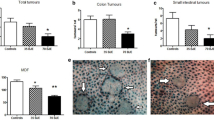
Açai, fruit from Euterpe oleraceae Martius, is consumed in natura and in a variety of beverages and food preparations and possesses several potential antioxidant compounds. In a first study for anticarcinogenicity screening, male Swiss mice (n = 20/per group) were chemically-induced to urothelial bladder carcinogenesis for 10 weeks and received a standard diet or a standard diet containing 2.5 and 5 % spray-dried açai pulp (AP) for 10 weeks. At week 20, the incidence of simple and nodular hyperplasia and the incidence and multiplicity of transitional cell carcinoma (TCC) were evaluated. In a second study for antigenotoxicity screening, male Swiss mice (n = 6/per group) were fed standard diet or standard diet containing 5 % AP for three weeks. Urothelial cell suspensions were obtained and challenged with H2O2 for induction of DNA damage and analyzed by comet assay. Overall, dietary 5 % AP reduced TCC incidence and multiplicity (p = 0.019 and p = 0.015, respectively) and tumor cell proliferation and p63 expression (p = 0.02 and p = 0.007, respectively), Furthermore, the group fed the 5 % AP presented a significant reduction (p < 0.01) in DNA damage induced by H2O2, a notable oxidant agent. The results suggest that the spray-dried açai pulp used here inhibits the TCC development in male Swiss mice, probably due to its potential antioxidant action.


Similar content being viewed by others
References
Keegan KA, Clark PE (2011) Bladder cancer. Curr Opin Oncol 23:275–282
Volanis D, Kadiyska T, Galanis A, Delakas D, Logotheti S, Zoumpourlis V (2010) Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicol Lett 193:131–137
Tanaka T, Miyazawa K, Tsukamoto T, Kuno T, Suzuki K (2011) Pathobiology and chemoprevention of bladder cancer. J Oncol. doi:10.1155/2011/528353
Steele VE, Lubet RA (2010) The use of animal models for cancer chemoprevention drug development. Semin Oncol 37:327–338
Poulose SM, Fisher DR, Larson J, Bielinski DF, Rimando AM, Carey AN, Schauss AG, Shukitt-Hale B (2012) Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem 60:1084–1093
Rodrigues BR, Lichtenthaler R, Zimmermann BF, Papagiannopoulos M, Fabricius H, Marx F (2006) Total oxidant scavenging capacity of Euterpe oleracea Mart. (Açai) seeds and identification of their polyphenolic compounds. J Agric Food Chem 54:4162–4167
Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, Derendorf H (2008) Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich Açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem 56:7796–7802
Schauss AG, Clewell A, Balogh L, Szakonyi IP, Financsek I, Horváth J, Thuroczy J, Béres E, Vértesi A, Hirka G (2010) Safety evaluation of an açai-fortified fruit and berry functional juice beverage (MonaVie Active®). Toxicol 278:46–54
Spada PDS, Souza GGN, Bortolini GV, Henriques JAP, Salvador M (2008) Antioxidant, mutagenic, and antimutagenic activity of frozen fruits. J Med Food 11:144–151
Kang J, Li Z, Wu T, Jensen GS, Schauss AG, Wu X (2010) Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem 122:610–617
Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, Scherwitz L, Beaman R, Endres JR, Schauss AG (2008) In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem 56:8326–8333
Ribeiro JC, Antunes LM, Aissa AF, Darin JD, De Rosso VV, Mercadante AZ, Bianchi Mde L (2010) Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat Res 695:22–28
Stoner GD, Wang LS, Seguin C, Rocha C, Stoner K, Chiu S, Kinghorn AD (2010) Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm Res 27:1138–1145
Szajdek A, Borowska EJ (2008) Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum Nutr 63:147–156
Paredes-López O, Cervantes-Ceja ML, Vigna-Pérez M, Hernández-Pérez T (2010) Berries: improving human health and healthy aging, and promoting quality life- A review. Plant Foods Hum Nutr 65:299–308
Bidinotto LT, Spinardi-Barbisan AL, Rocha NS, Salvadori DM, Barbisan LF (2006) Effects of ginger (Zingiber officinale Roscoe) on DNA damage and development of urothelial tumors in a mouse bladder carcinogenesis model. Environ Mol Mutagen 47:624–630
Cohen SM (2002) Comparative pathology of proliferative lesions of the urinary bladder. Toxicol Pathol 30:663–671
Bidinotto LT, Costa CA, Costa M, Rodrigues MA, Barbisan LF (2012) Modifying effects of lemongrass essential oil on specific tissue response to the carcinogen N-methyl-N-nitrosurea in female BALB/c mice. J Med Food 15:161–168
Miranda DD, Arçari DP, Pedrazzoli J Jr, Carvalho Pde O, Cerutti SM, Bastos DH, Ribeiro ML (2008) Protective effects of mate tea (Ilex paraguariensis) on H2O2-induced DNA damage and DNA repair in mice. Mutagenesis 23:261–265
Cohen SM, Ohnishi T, Clark NM, He J, Arnold LL (2007) Investigations of rodent urinary bladder carcinogens: Collection, processing, and evaluation of urine and bladders. Toxicol Pathol 35:337–347
Karni-Schmidt O, Castillo-Martin M, Shen TH, Gladoun N, Domingo-Domenech J, Sanchez-Carbayo M, Li Y, Lowe S, Prives C, Cordon-Cardo C (2011) Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am J Pathol 178:1350–1360
Buza N, Cohen PJ, Hui P, Parkash V (2010) Inverse p16 and p63 expression in small cell carcinoma and high-grade urothelial cell carcinoma of the urinary bladder. Int J Surg Pathol 18:94–102
Shiina H, Igawa M, Urakami S, Shigeno K, Yoneda T, Terashima M, Deguchi M, Ribeiro-Filho L, Dahiya R (2001) Alterations of beta- and gamma-catenin in N-butyl-N-(-4-hydroxybutyl)nitrosamine-induced murine bladder cancer. Cancer Res 61:7101–7109
Hu X, Ruan Y, Cheng F, Yu W, Zhang X, Larré S (2011) p130Cas, E-cadherin and β-catenin in human transitional cell carcinoma of the bladder: Expression and clinicopathological significance. Int J Urol 18:630–637
Ziech D, Franco R, Pappa A, Panayiotidis MI (2011) Reactive oxygen species (ROS) induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res 711:167–173
Hempel N, Ye H, Abessi B, Mian B, Melendez JA (2009) Altered redox status accompanies progression to metastatic human bladder cancer. Free Radic Biol Med 46:42–50
Acknowledgments
This study was supported by CAPES. Mariana F. Fragoso and Monize G do Prado and Luis F. Barbisan were recipients of fellowships from CAPES, FAPESP (07/54858-5, 09/50890-7) or CNPq (301585/2009-1), respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
General and biochemical parameters evaluated in carcinogens-treated groups.1 (DOC 42 kb)
Supplementary Figure
Histopathology of urothelium detected in the HEstained sections. A) Control group (standard diet) and group receiving 0.5% AP showing non-altered urothelium (20x objective), B-E) BBN/MNU-treated groups showing simple and nodular hyperplasia (B-C, 20x objective) and TCC showing invasion on muscularis (D, detailed in E). (PPT 24718 kb)
Rights and permissions
About this article
Cite this article
Fragoso, M.F., Prado, M.G., Barbosa, L. et al. Inhibition of Mouse Urinary Bladder Carcinogenesis by Açai Fruit (Euterpe oleraceae Martius) Intake. Plant Foods Hum Nutr 67, 235–241 (2012). https://doi.org/10.1007/s11130-012-0308-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0308-y