Abstract
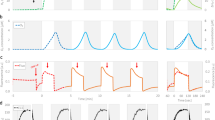
Over 40 years ago, Joliot et al. (Photochem Photobiol 10:309–329, 1969) designed and employed an elegant and highly sensitive electrochemical technique capable of measuring O2 evolved by photosystem II (PSII) in response to trains of single turn-over light flashes. The measurement and analysis of flash-induced oxygen evolution patterns (FIOPs) has since proven to be a powerful method for probing the turnover efficiency of PSII. Stemler et al. (Proc Natl Acad Sci USA 71(12):4679–4683, 1974), in Govindjee’s lab, were the first to study the effect of “bicarbonate” on FIOPs by adding the competitive inhibitor acetate. Here, we extend this earlier work by performing FIOPs experiments at various, strictly controlled inorganic carbon (Ci) levels without addition of any inhibitors. For this, we placed a Joliot-type bare platinum electrode inside a N2-filled glove-box (containing 10–20 ppm CO2) and reduced the Ci concentration simply by washing the samples in Ci-depleted media. FIOPs of spinach thylakoids were recorded either at 20-times reduced levels of Ci or at ambient Ci conditions (390 ppm CO2). Numerical analysis of the FIOPs within an extended Kok model reveals that under Ci-depleted conditions the miss probability is discernibly larger (by 2–3 %) than at ambient conditions, and that the addition of 5 mM HCO3 − to the Ci-depleted thylakoids largely restores the original miss parameter. Since a “mild” Ci-depletion procedure was employed, we discuss our data with respect to a possible function of free or weakly bound HCO3 − at the water-splitting side of PSII.



Similar content being viewed by others
Abbreviations
- α:
-
Miss parameter
- β:
-
Double hit parameter
- Ci :
-
Inorganic carbon (HCO3 −, CO2, CO3 2−)
- C −i :
-
Inorganic carbon depleted
- C +i :
-
Containing ambient level of inorganic carbon
- FIOP:
-
Flash-induced oxygen evolution pattern
- MIMS:
-
Membrane-inlet mass spectrometry
- PSII:
-
Photosystem II
- OEC:
-
Oxygen-evolving complex
- S i states:
-
Oxidation states of the OEC, where i is the number of stored oxidizing equivalents
References
Allakhverdiev SI, Yruela I, Picorel R, Klimov VV (1997) Bicarbonate is an essential constituent of the water-oxidizing complex of photosystem II. Proc Natl Acad Sci USA 94(10):5050–5054
Ananyev G, Dismukes GC (2005) How fast can photosystem II split water? Kinetic performance at high and low frequencies. Photosynth Res 84(1):355–365
Ananyev G, Nguyen T, Putnam-Evans C, Dismukes GC (2005) Mutagenesis of CP43-arginine-357 to serine reveals new evidence for (bi)carbonate functioning in the water oxidizing complex of photosystem II. Photochem Photobiol Sci 4(12):991–998
Aoyama C, Suzuki H, Sugiura M, Noguchi T (2008) Flash-induced FT-IR difference spectroscopy shows no evidence for the structural coupling of bicarbonate to the oxygen-evolving Mn cluster in photosystem II. Biochemistry 47(9):2760–2765
Baranov SV, Ananyev GM, Klimov VV, Dismukes GC (2000) Bicarbonate accelerates assembly of the inorganic core of the water-oxidizing complex in manganese depleted photosystem II: a proposed biogeochemical role for atmospheric carbon dioxide in oxygenic photosynthesis. Biochemistry 39(20):6060–6065
Baranov SV, Tyryshkin AM, Katz D, Dismukes GC, Ananyev GM, Klimov VV (2004) Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry 43(7):2070–2079
Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W (2009) On-line mass spectrometry: membrane inlet sampling. Photosynth Res 102:511–522
Berthomieu C, Hienerwadel R (2001) Iron coordination in photosystem II: interaction between bicarbonate and QB pocket studied by Fourier transform infrared spectroscopy. Biochemistry 40:4044–4052
Cardona T, Sedoud A, Cox N, Rutherford AW (2012) Charge separation in Photosystem II: a comparative and evolutionary overview. Biochim Biophys Acta 1817(1):26–43
Christen G, Seeliger A, Renger G (1999) P680+· reduction kinetics and redox transition probability of the water oxidizing complex as a function of pH and H/D isotope exchange in spinach thylakoids. Biochemistry 38(19):6082–6092
Clausen J, Beckmann K, Junge W, Messinger J (2005) Evidence that bicarbonate is not the substrate in photosynthetic oxygen evolution. Plant Physiol 139(3):1444–1450
Cox N, Messinger J (2013) Reflections on substrate water and dioxygen formation. Biochim Biophys Acta. doi:10.1016/j.bbabio.2013.01.013 (in press)
Cox N, Jin L, Jaszewski A, Smith PJ, Krausz E, Rutherford AW, Pace R (2009) The semiquinone-iron complex of photosystem II: structural insights from ESR and theoretical simulation; evidence that the native ligand to the non-heme iron is carbonate. Biophys J 97(7):2024–2033
Dasgupta J, Tyryshkin AM, Dismukes GC (2007) ESEEM spectroscopy reveals carbonate and an N-donor protein-ligand binding to Mn2+ in the photoassembly reaction of the Mn4Ca cluster in photosystem II. Angew Chem Int Ed 46(42):8028–8031
Dasgupta J, Ananyev GM, Dismukes GC (2008) Photoassembly of the water-oxidizing complex in photosystem II. Coord Chem Rev 252(3–4):347–360
Dasgupta J, Tyryshkin AM, Baranov SV, Dismukes GC (2010) Bicarbonate coordinates to Mn3+ during photo-assembly of the catalytic Mn4Ca core of photosynthetic water oxidation: EPR characterization. Appl Magn Reson 37(1–4):137–150
Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle: basic framework and possible realization at the atomic level. Coord Chem Rev 252(3–4):273–295
Dau H, Zaharieva I, Haumann M (2012) Recent developments in research on water oxidation by photosystem II. Curr Opin Chem Biol 16(1–2):3–10
de Wijn R, van Gorkom HJ (2002) S-state dependence of the miss probability in photosystem II. Photosynth Res 72(2):217–222
Diner BA (1977) Dependence of deactivation reactions of photosystem II on redox state of plastoquinone pool A varied under anaerobic conditions. Equilibria on the acceptor side of photosystem II. Biochim Biophys Acta 460:247–258
Diner BA, Petrouleas V (1990) Formation by NO of nitrosyl adducts of redox components of the photosystem II reaction center. 2. Evidence that HCO3 −/CO2 binds to the acceptor-side non-heme iron. Biochim Biophys Acta 1015(1):141–149
El-Shintinawy F, Govindjee (1990) Bicarbonate effect in leaf discs from spinach. Photosynth Res 24:189–200
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303(5665):1831–1838
Forbush B, Kok B, McGloin MP (1971) Cooperation of charges in photosynthetic oxygen evolution. II. Damping of flash yield oscillation, deactivation. Photochem Photobiol 14(3):307–321
Fromme R, Hagemann R, Renger G (1987) Comparative studies of electron transport and atrazine binding in thylakoids and PS II particles from spinach. In: Biggens J (ed) Progress in Photosynthesis Research, vol III. Martinus Nijhoff Publishers, Dordrecht, pp 783–786
Govindjee, Xu C, van Rensen JJS (1997) On the requirement of bound bicarbonate for photosystem II activity. Z Naturforsch 52(1–2):24–32
Govindjee Weger HG, Turpin DH, van Rensen JJS, Devos OJ, Snel JFH (1991) Formate releases carbon dioxide/bicarbonate from thylakoid membranes: measurements by mass spectroscopy and infrared gas analyzer. Naturwissenschaften 78(4):168–170
Govindjee, Kern J, Messinger J, Whitmarsh J (2010) Photosystem II. In: Encyclopedia of Life Sciences (ELS). Wiley, Chichester. doi:10.1002/9780470015902.a0000669.pub2
Guskov A, Gabdulkhakov A, Broser M, Glöckner C, Hellmich J, Kern J, Frank M, Saenger W, Zouni A (2010) Recent progress in the crystallographic studies of photosystem II. ChemPhysChem 11:1160–1171
Han GY, Mamedov F, Styring S (2012) Misses during water oxidation in photosystem II are S state-dependent. J Biol Chem 287(16):13422–13429
Hienerwadel R, Berthomieu C (1995) Bicarbonate binding to the non-heme iron of photosystem II investigated by Fourier transform infrared difference spectroscopy and 13C-labeled bicarbonate. Biochemistry 34:16288–16297
Hillier W, Messinger J (2005) Mechanism of photosynthetic oxygen production. In: Wydrzynski T, Satoh K (eds) Photosystem II. The Light-Driven water:plastoquinone oxidoredutase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 567–608
Hillier W, McConnell I, Badger MR, Boussac A, Klimov VV, Dismukes GC, Wydrzynski T (2006) Quantitative assessment of intrinsic carbonic anhydrase activity and the capacity for bicarbonate oxidation in photosystem II. Biochemistry 45(7):2094–2102
Isgandarova S, Renger G, Messinger J (2003) Functional differences of photosystem II from Synechococcus elongatus and spinach characterized by flash-induced oxygen evolution patterns. Biochemistry 42(30):8929–8938
Joliot P (1972) Modulated light source use with the oxygen electrode. In: San Pietro A (ed) Photosynthesis and nitrogen fixation, vol 24 B. Methods of enzymology. Academic Press, New York, pp 123–134
Joliot P (2003) Period-four oscillations of the flash-induced oxygen formation in photosynthesis. Photosynth Res 76(1–3):65–72
Joliot P, Barbieri G, Chabaud R (1969) Un nouveau modele des centres photochimiques du systeme II. Photochem Photobiol 10:309–329
Jursinic P (1981) Investigation of double turnovers in photosystem II charge separation and oxygen evolution with excitation flashes of different duration. Biochim Biophys Acta 635:38–52
Jursinic P, Stemler A (1982) A seconds range component of the reoxidation of the primary photosystem II acceptor Q: effects of bicarbonate depletion in chloroplasts. Biochim Biophys Acta 681(3):419–428
Jursinic PA, Stemler A (1984) Effects of bicarbonate depletion on secondary acceptors of photosystem II. Biochim Biophys Acta 764(2):170–178
Kebekus U, Messinger J, Renger G (1995) Structural changes in the water oxidizing complex monitored via the pH dependence of the reduction rate of redox state S1 by hydrazine and hydroxylamine in isolated spinach thylakoids. Biochemistry 34:6175–6182
Klimov VV, Allakhverdiev SI, Baranov SV, Feyziev YM (1995a) Effects of bicarbonate and formate on the donor side of photosystem 2. Photosynth Res 46(1–2):219–225
Klimov VV, Allakhverdiev SI, Feyziev YM, Baranov SV (1995b) Bicarbonate requirement for the donor side of photosystem II. FEBS Lett 363(3):251–255
Klimov VV, Hulsebosch RJ, Allakhverdiev SI, Wincencjusz H, van Gorkom HJ, Hoff AJ (1997) Bicarbonate may be required for ligation of manganese in the oxygen-evolving complex of photosystem II. Biochemistry 36(51):16277–16281
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution. Photochem Photobiol 11:457–476
Konermann L, Messinger J, Hillier W (2008) Mass spectrometry based methods for studying kinetics and dynamics in biological systems. In: Amesz J, Hoff AJ (eds) Biophysical techniques in photosynthesis, vol 26., Series advances in photosynthesis and respirationSpringer, Dordrecht, pp 167–190
Kouřil R, Dekker JP, Boekema EJ (2012) Supramolecular organization of photosystem II in green plants. Biochim Biophys Acta 1817(1):2–12
Krishtalik LI (2000) The mechanism of the proton transfer: an outline. Biochim Biophys Acta 1458(1):6–27
Mar T, Govindjee (1972) Kinetic models of oxygen evolution in photosynthesis. J Theoret Biol 36:427–446
McConnell IL, Eaton-Rye JJ, Van Rensen JJS (2012) Regulation of photosystem II electron transport by bicarbonate. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD (eds) Photosynthesis: plastid biology, energy conversion and carbon assimilation. Springer, Dordrecht, pp 475–500
Mende D, Wiessner W (1985) Bicarbonate in vivo requirement of photosystem II in the green alga Chlamydobotrys stellata. J Plant Physiol 118(3):259–266
Messinger J (1993) Untersuchungen über die reaktiven Eigenschaften der verschiedenen Redoxzustände der Wasseroxidase Höherer Pflanzen. TU Berlin, Berlin
Messinger J, Renger G (1990) The reactivity of hydrazine with PS II strongly depends on the redox state of the water oxidizing system. FEBS Lett 277:141–146
Messinger J, Renger G (1993) Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox States S0, S−1 and S−2 of the water oxidase in isolated spinach thylakoids. Biochemistry 32(36):9379–9386
Messinger J, Renger G (2008) Photosynthetic water splitting. In: Renger G (ed) Primary processes of photosynthesis, part 2 principles and apparatus, vol 9., Comprehensive series in photochemical and photobiological sciencesRSC Publishing, Cambridge, pp 291–351
Messinger J, Schröder WP, Renger G (1993) Structure-function relations in photosystem II. Effects of temperature and chaotropic agents on the period four oscillation of flash induced oxygen evolution. Biochemistry 32:7658–7668
Messinger J, Badger MR, Wydrzynski T (1995) Detection of one slowly exchanging substrate water molecule in the S3 state of photosystem II. Proc Natl Acad Sci USA 92:3209–3213
Messinger J, Seaton G, Wydrzynski T, Wacker U, Renger G (1997) S−3 state of the water oxidase in photosystem II. Biochemistry 36:6862–6873
Metzner H (1978) Photosynthetic oxygen evolution. Academic Press, London
Müh F, Glöckner C, Hellmich J, Zouni A (2012) Light-induced quinone reduction in photosystem II. Biochim Biophys Acta 1817(1):44–65
Nöring B, Shevela D, Renger G, Messinger J (2008) Effects of methanol on the Si-state transitions in photosynthetic water-splitting. Photosynth Res 98:251–260
Nugent JHA, Demetriou C, Lockett CJ (1987) Electron donation in photosystem II. Biochim Biophys Acta 894(3):534–542
Pobeguts OV, Smolova TN, Timoshevsky DS, Klimov VV (2010) Interaction of bicarbonate with the manganese-stabilizing protein of photosystem II. J Photochem Photobiol B 100:30–37
Renger G (2010) The light reactions of photosynthesis. Curr Sci 98:1305–1319
Renger G, Hanssum B (1988) Studies on the deconvolution of flash induced absorption changes into the difference spectra of individual redox steps within the water oxidizing enzyme system. Photosynth Res 16:243–259
Renger G, Hanssum B (2009) Oxygen detection in biological systems. Photosynth Res 102(2–3):487–498
Renger G, Holzwarth AR (2005) Primary electron transfer. In: Wydrzynski TJ, Satoh K (eds) Photosystem II. The light-driven water: plastoquinone oxidoreductase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 139–175
Saito K, Rutherford AW, Ishikita H (2013) Mechanism of proton-coupled quinone reduction in photosystem II. Proc Natl Acad Sci USA 110(3):954–959
Shevela D, Messinger J (2012) Probing the turnover efficiency of photosystem II membrane fragments with different electron acceptors. Biochim Biophys Acta 1817(8):1208–1212
Shevela D, Nöring B, Eckert HJ, Messinger J, Renger G (2006a) Characterization of the water oxidizing complex of photosystem II of the Chl d-containing cyanobacterium Acaryochloris marina via its reactivity towards endogenous electron donors and acceptors. Phys Chem Chem Phys 8(29):3460–3466
Shevela DN, Khorobrykh AA, Klimov VV (2006b) Effect of bicarbonate on the water-oxidizing complex of photosystem II in the super-reduced S-states. Biochim Biophys Acta 1757(4):253–261
Shevela D, Klimov V, Messinger J (2007) Interactions of photosystem II with bicarbonate, formate and acetate. Photosynth Res 94(2–3):247–264
Shevela D, Klimov V, Messinger J (2008a) Formate-induced release of carbon dioxide/hydrogen carbonate from photosystem II. In: Allen JF, Gantt E, Golbeck JH, Osmond B (eds) Photosynthesis. Energy from the Sun. Springer, Glasgow, pp 497–501
Shevela D, Su JH, Klimov V, Messinger J (2008b) Hydrogen carbonate is not a tightly bound constituent of the water-oxidizing complex in photosystem II. Biochim Biophys Acta 1777(6):532–539
Shevela D, Eaton-Rye JJ, Shen J-R, Govindjee (2012) Photosystem II and the unique role of bicarbonate: a historical perspective. Biochim Biophys Acta 1817(8):1134–1151
Shinkarev V (2005) Flash induced oxygen evolution and other oscillatory processes. In: Wydrzynski T, Satoh K (eds) Photosystem II. The light-driven water: plastoquinone oxidoredutase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 539–565
Shinkarev V, Wraight CA (1993) Oxygen evolution in photosynthesis: from unicycle to bicycle. Proc Natl Acad Sci USA 90:1834–1838
Shutova T, Kenneweg H, Buchta J, Nikitina J, Terentyev V, Chernyshov S, Andersson B, Allakhverdiev SI, Klimov VV, Dau H, Junge W, Samuelsson G (2008) The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J 27(5):782–791
Siegbahn PEM, Lundberg M (2006) Hydroxide instead of bicarbonate in the structure of the oxygen evolving complex. J Inorg Biochem 100(5–6):1035–1040
Stemler A (1980) Forms of dissolved carbon dioxide required for photosystem II activity in chloroplast membranes. Plant Physiol 65(6):1160–1165
Stemler A (1982) The functional role of bicarbonate in photosynthetic light reaction II. In: Govindjee (ed) Photosynthesis vol II. Academic Press, New York, pp 513–538
Stemler AJ (2002) The bicarbonate effect, oxygen evolution, and the shadow of Otto Warburg. Photosynth Res 73(1–3):177–183
Stemler A, Govindjee (1973) Bicarbonate ion as a critical factor in photosynthetic oxygen evolution. Plant Physiol 52(2):119–123
Stemler AJ, Lavergne J (1997) Evidence that formate destabilizes the S−1 state of the oxygen-evolving mechanism in Photosystem II. Photosynth Res 51(2):83–92
Stemler A, Babcock GT, Govindjee (1974) Effect of bicarbonate on photosynthetic oxygen evolution in flashing light in chloroplast fragments. Proc Natl Acad Sci USA 71(12):4679–4683
Styring S, Rutherford AW (1987) In the oxygen evolving complex of photosystem II the S0 state is oxidized to the S1 State by Y +D (Signal IIslow). Biochemistry 26:2401–2405
Suzuki H, Sugiura M, Noguchi T (2012) Determination of the miss probabilities of individual S-state transitions during photosynthetic water oxidation by monitoring electron flow in photosystem II using FTIR spectroscopy. Biochemistry 51(34):6776–6785
Ulas G, Brudvig GW (2010) Zwitterion modulation of O2-evolving activity of cyanobacterial photosystem II. Biochemistry 49(37):8220–8227
Ulas G, Olack G, Brudvig GW (2008) Evidence against bicarbonate bound in the O2-evolving complex of photosystem II. Biochemistry 47(10):3073–3075
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Van Rensen JJS (2002) Role of bicarbonate at the acceptor side of photosystem II. Photosynth Res 73(1–3):185–192
Van Rensen JJS, Klimov VV (2005) Bicarbonate interactions. In: Wydrzynski T, Satoh K (eds) Photosystem II. The light-driven water: plastoquinone oxidoreductase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 329–346
Van Rensen JJS, Xu C, Govindjee (1999) Role of bicarbonate in photosystem II, the water-plastoquinone oxido-reductase of plant photosynthesis. Physiol Plant 105:585–592
Vass I, Deak Z, Hideg E (1990a) Charge equilibrium between the water oxidizing complex and the electron donor tyrosine D in photosystem II. Biochim Biophys Acta 1017:63–69
Vass I, Deak Z, Jegerschold C, Styring S (1990b) The accessory electron-donor tyrosine D of photosystem II is slowly reduced in the dark during low-temperature storage of isolated thylakoids. Biochim Biophys Acta 1018(1):41–46
Vermaas WEJ, Renger G, Dohnt G (1984) The reduction of the oxygen evolving system in chloroplasts by thylakoid components. Biochim Biophys Acta 764(2):194–202
Villarejo A, Shutova T, Moskvin O, Forssen M, Klimov VV, Samuelsson G (2002) A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J 21(8):1930–1938
Warburg O (1964) Prefactory chapter. Annu Rev Biochem 33:1–18
Warburg O, Krippahl G (1958) Hill-Reaktionen. Z Naturforsch B 13(8):509–514
Winget GD, Izawa S, Good NE (1965) Stoichiometry of photophosphorylation. Biochem Biophys Res Commun 21(5):438–441
Wydrzynski T, Govindjee (1975) New site of bicarbonate effect in photosystem II of photosynthesis: evidence from chlorophyll fluorescence transients in spinach-chloroplasts. Biochim Biophys Acta 387(2):403–408
Wydrzynski T, Satoh K (eds) (2005) Photosystem II. The light-driven water: plastoquinone oxidoreductase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht
Yano J, Kern J, Sauer K, Latimer MJ, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK (2006) Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314:821–825
Zaharieva I, Wichmann JM, Dau H (2011) Thermodynamic limitations of photosynthetic water oxidation at high proton concentrations. J Biol Chem 286(20):18222–18228
Acknowledgments
This study was supported by the Knut and Alice Wallenberg Foundation, the Kempe Foundation, the Swedish Research Council (VR), the Strong Research Environment Solar Fuels (Umeå University), the Artificial Leaf Project (K&A Wallenberg Foundation) and the Max-Planck Gesellschaft. The authors would like to thank Govindjee, Vyacheslav Klimov, and Alan Stemler for fruitful discussions on the “bicarbonate effect” over the last few years and Ethel Hüttel for sample preparation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shevela, D., Nöring, B., Koroidov, S. et al. Efficiency of photosynthetic water oxidation at ambient and depleted levels of inorganic carbon. Photosynth Res 117, 401–412 (2013). https://doi.org/10.1007/s11120-013-9875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9875-5