Abstract
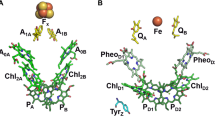
The primary electron donor (P) in the photosynthetic bacterial reaction center of Rhodobacter sphaeroides and Blastochloris viridis consists of a dimer of bacteriochlorophyll a and b cofactors, respectively. Its photoexcited triplet state in frozen solution has been investigated by time resolved ENDOR spectroscopy at 34 GHz. The observed ENDOR spectra for 3P865 and 3P960 are essentially the same, indicating very similar spin density distributions. Exceptions are the ethylidene groups unique to the bacteriochlorophyll b dimer in 3P960. Strikingly, the observed hyperfine coupling constants of the ethylidene groups are larger than in the monomer, which speaks for an asymmetrically delocalized wave function over both monomer halves in the dimer. The latter observation corroborates previous findings of the spin density in the radical cation states P •+865 (Lendzian et al. in Biochim Biophys Acta 1183:139–160, 1993) and P •+960 (Lendzian et al. in Chem Phys Lett 148:377–385, 1988). As compared to the bacteriochlorophyll monomer, the hyperfine coupling constants of the methyl groups 21 and 121 are reduced by at least a factor of two, and quantitative analysis of these couplings gives rise to a ratio of approximately 3:1 for the spin density on the halves PL:PM. Our findings are discussed in light of the large difference in photosynthetic activity of the two branches of cofactors present in the bacterial reaction center proteins.







Similar content being viewed by others
Notes
In an alternate description, the triplet state of the primary donor can be described as a combination of two local excitations 3PLPM, P 3L PM and charge transfer states P + L P − M and P − L P + M ; however, the weight contribution of individual terms in the wavefunction is unknown (Lendzian et al. 1998; Thurnauer 1979).
Abbreviations
- Bl. :
-
Blastochloris
- bRC:
-
Bacterial reaction center
- BChl a :
-
Bacteriochlorophyll a
- BChl b :
-
Bacteriochlorophyll b
- BPheo:
-
Bacteriopheophytin
- DFT:
-
Density functional theory
- EPR:
-
Electron paramagnetic resonance
- ENDOR:
-
Electron nuclear double resonance
- GGA:
-
Generalized gradient approximation
- HOMO:
-
Highest occupied molecular orbital
- LUMO:
-
Lowest unoccupied molecular orbital
- P:
-
Primary donor
- P865 :
-
Primary donor in Rhodobacter sphaeroides
- P960 :
-
Primary donor in Blastochloris viridis
- Q:
-
Quinone
- Rb. :
-
Rhodobacter
- SCF:
-
Self-consistent field
- ZFS:
-
Zero field splitting
References
Allen JP, Feher G (1984) Crystallization of reaction center from Rhodopseudomonas sphaeroides—preliminary characterization. Proc Natl Acad Sci U S A 81(15):4795–4799
Allen JP, Feher G, Yeates TO, Rees DC, Deisenhofer J, Michel H, Huber R (1986) Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by X-Ray-diffraction. Proc Natl Acad Sci U S A 83(22):8589–8593
Allen JP, Feher G, Yeates TO, Komiya H, Rees DC (1987a) Structure of the reaction center from Rhodobacter sphaeroides R-26—the cofactors. Proc Natl Acad Sci U S A 84(16):5730–5734
Allen JP, Feher G, Yeates TO, Komiya H, Rees DC (1987b) Structure of the reaction center from Rhodobacter sphaeroides R-26—the protein subunits. Proc Natl Acad Sci U S A 84(17):6162–6166
Allen JP, Feher G, Yeates TO, Komiya H, Rees DC (1988a) Structure of the reaction center from Rhodobacter sphaeroides R-26—protein cofactor (Quinones and Fe2+) interactions. Proc Natl Acad Sci U S A 85(22):8487–8491
Allen JP, Feher G, Yeates TO, Komiya H, Rees DC (1988b) Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1. In: Breton J, Vermeglio A (eds) The photosynthetic bacterial reaction center. Plenum Press, New York, pp 5–11
Angerhofer A (1991) Chlorophyll triplets and radical pairs. In: Scheer H (ed) Chlorophylls. CRC Press, Boca Raton, pp 945–991
Atherton N (1993) Principles of electron spin resonance. Ellis Horwood PTR Prentice Hall, New York
Baxter RHG, Seagle BL, Ponomarenko N, Norris JR (2005) Cryogenic structure of the photosynthetic reaction center of Blastochloris viridis in the light and dark. Acta Crystallogr Sect D: Biol Crystallogr 61:605–612. doi:10.1107/s0907444905005809
Blankenship R (2002) Molecular mechanisms of photosynthesis. Blackwell, Oxford, London
Blankenship B, Madigan M, Bauer C (1995) Anoxygenic photosynthetic bacteria. Advances in photosynthesis, vol 2. Kluwer, Dordrecht
Borg DC, Forman A, Fajer J (1976) ESR and ENDOR studies of Pi-cation radical of bacteriochlorophyll. J Am Chem Soc 98(22):6889–6893
Breton J, Vermeglio A (1988) The photosynthetic bacterial reaction center—structure and dynamics. Plenum Press, New York
Breton J, Vermeglio A (1992) The photosynthetic bacterial reaction center II. Plenum Press, New York
Budil DE, Taremi SS, Gast P, Norris JR, Frank HA (1988) Single-crystal electron-spin resonance studies of the photochemical-reaction center from Rhodobacter sphaeroides wild-type strain 2.4.1. Isr J Chem 28(2–3):59–66
Carrington A, McLachlan AD (1967) Introduction to magnetic resonance. A Harper International Edition. Harper & Row, John Weatherhill Inc., New York, Tokyo
Chang CH, El-kabbani O, Tiede D, Norris J, Schiffer M (1991) Structure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides. Biochemistry 30(22):5352–5360. doi:10.1021/bi00236a005
Clayton RK, Sistrom WR (1978) The photosynthetic bacteria. Plenum Press, New York
Davis MS, Forman A, Hanson LK, Thornber JP, Fajer J (1979) Anion and cation radicals of bacteriochlorophyll and bacteriopheophytin b—their role in the primary charge separation of Rhodopseudomonas viridis. J Phys Chem 83(26):3325–3332
Deisenhofer J, Norris JR (1993) The photosynthetic reaction center, vol I and II. Academic Press, San Diego
Deisenhofer J, Epp O, Miki K, Huber R, Michel H (1984) X-ray structure-analysis of a membrane-protein complex—electron-density map at 3Å resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol 180(2):385–398
Deisenhofer J, Epp O, Miki K, Huber R, Michel H (1985) Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3Å resolution. Nature 318(6047):618–624
Ermler U, Fritzsch G, Buchanan SK, Michel H (1994) Structure of the photosynthetic reaction-center from Rhodobacter sphaeroides at 2.65-Ångström resolution—cofactors and protein-cofactor interactions. Structure 2(10):925–936
Fajer J, Borg DC, Forman A, Dolphin D, Felton RH (1973) Anion radical of bacteriochlorophyll. J Am Chem Soc 95(8):2739–2741
Feher G, Okamura MY (1978) Chemical composition and properties of reaction centers. In: Clayton R, Sistrom W (eds) The photosynthetic bacteria. Plenum Press, New York, pp 349–386
Feher G, Allen JP, Okamura MY, Rees DC (1989) Structure and function of bacterial photosynthetic reaction centers. Nature 339(6220):111–116
Frank HA, Bolt JD, Costa SMDB, Sauer K (1980) Electron-paramagnetic resonance detection of carotenoid triplet-states. J Am Chem Soc 102(15):4893–4898
Frank HA, Chynwat V, Hartwich G, Meyer M, Katheder I, Scheer H (1993) Carotenoid triplet-state formation in Rhodobacter sphaeroides R-26 reaction centers exchanged with modified bacteriochlorophyll pigments and reconstituted with spheroidene. Photosynth Res 37(3):193–203
Ganyushin D, Neese F (2006) First-principles calculations of zero-field splitting parameters. J Chem Phys 125(2):024103
Gardiner AT, Zech SG, MacMillan F, Käß H, Bittl R, Schlodder E, Lendzian F, Lubitz W (1999) Electron paramagnetic resonance studies of zinc-substituted reaction centers from Rhodopseudomonas viridis. Biochemistry 38(36):11773–11787
Gouterman M (1959) Study of the effects of substitution on the absorption spectra of porphin. J Chem Phys 30(5):1139–1161
Gouterman M (1961) Spectra of porphyrins. J Mol Spectrosc 6(1):138–163
Hoff AJ, Proskuryakov II (1985) Triplet electron-paramagnetic-res spectra of the primary electron-donor in bacterial photosynthesis at temperatures between 15 K and 296 K. Chem Phys Lett 115(3):303–310
Hunter CN, Daldal F, Thurnauer MC, Beautty JT (2009) The purple phototrophic bacteria. Springer, Heidelberg
Katona G, Andreasson U, Landau EM, Andreasson LE, Neutze R (2003) Lipidic cubic phase crystal structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.35 ångström resolution. J Mol Biol 331(3):681–692. doi:10.1016/s0022-2836(03)00751-4
Koch W, Holthausen MC (1999) A chemist’s guide to density functional theory. Wiley-VCH, Weinheim
Koepke J, Krammer E-M, Klingen AR, Sebban P, Ullmann GM, Fritzsch G (2007) pH modulates the quinone position in the photosynthetic reaction center from Rhodobacter sphaeroides in the neutral and charge separated states. J Mol Biol 371(2):396–409. doi:10.1016/j.jmb.2007.04.082
Lancaster CRD, Hunte C, Kelley J III, Trumpower BL, Ditchfield R (2007) A comparison of stigmatellin conformations, free and bound to the photosynthetic reaction center and the cytochrome bc(1) complex. J Mol Biol 368(1):197–208. doi:10.1016/j.jmb.2007.02.013
Lendzian F, van Willigen H, Sastry S, Möbius K, Scheer H, Feick R (1985) Proton ENDOR study of the photoexcited triplet-state PT in Rps. sphaeroides R-26 photosynthetic reaction centers. Chem Phys Lett 118(2):145–150
Lendzian F, Lubitz W, Scheer H, Hoff AJ, Plato M, Tränkle E, Möbius K (1988) Electron-spin-resonance, ENDOR and TRIPLE resonance studies of the primary donor radical cation P +•960 in the photosynthetic bacterium Rhodopseudomonas viridis. Chem Phys Lett 148(5):377–385
Lendzian F, Huber M, Isaacson RA, Endeward B, Plato M, Bönigk B, Möbius K, Lubitz W, Feher G (1993) The electronic-structure of the primary donor cation-radical in Rhodobacter sphaeroides R-26—ENDOR and TRIPLE-resonance studies in single-crystals of reaction centers. Biochim Biophys Acta 1183(1):139–160
Lendzian F, Bittl R, Lubitz W (1998) Pulsed ENDOR of the photoexcited triplet states of bacteriochlorophyll a and of the primary donor P-865 in reaction centers of Rhodobacter sphaeroides R-26. Photosynth Res 55(2–3):189–197
Levanon H, Norris JR (1978) Photoexcited triplet-state and photosynthesis. Chem Rev 78(3):185–198
Li L, Mustafi D, Fu Q, Tereshko V, Chen DL, Tice JD, Ismagilov RF (2006) Nanoliter microfluidic hybrid method for simultaneous screening and optimization validated with crystallization of membrane proteins. Proc Natl Acad Sci USA 103(51):19243–19248. doi:10.1073/pnas.0607502103
Lubitz W (1991) EPR and ENDOR studies of chlorophyll cation and anion radicals. In: Scheer H (ed) The chlorophylls. CRC Press, Boca Raton, pp 903–944
Lubitz W, Lendzian F, Möbius K (1981) 14N and 1H electron nuclear multiple resonance experiments on bacteriochlorophyll a anion radicals in solution. Chem Phys Lett 81(2):235–241
Lubitz W, Lendzian F, Plato M, Scheer H, Möbius K (1997) The bacteriochlorophyll a cation radical revisited. An ENDOR and TRIPLE resonance study. Appl Magn Reson 13(3–4):531–551
Lubitz W, Lendzian F, Bittl R (2002) Radicals, radical pairs and triplet states in photosynthesis. Acc Chem Res 35(5):313–320. doi:10.1021/ar000084g
Marchanka A, Paddock M, Lubitz W, van Gastel M (2007) Low-temperature pulsed EPR study at 34 GHz of the Triplet states of the primary electron donor P865 and the carotenoid in native and mutant bacterial reaction centers of Rhodobacter sphaeroides. Biochemistry 46:14782–14794
Marchanka A, Lubitz W, van Gastel M (2009) Spin density distribution on the excited triplet state of bacteriochlorophyll. Pulsed ENDOR and DFT studies. J Phys Chem B 113:6917–6927
Michel H (1982) 3-Dimensional crystals of a membrane-protein complex—the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol 158(3):567–572
Michel-Beyerle ME (1990) Reaction centers of photosynthetic bacteria. Springer, Berlin
Michel-Beyerle ME (1996) Reaction centers of photosynthetic bacteria—structure and dynamics. Springer, Berlin
Michel-Beyerle ME, Plato M, Deisenhofer J, Michel H, Bixon M, Jortner J (1988) Unidirectionality of charge separation in reaction centers of photosynthetic bacteria. Biochim Biophys Acta 932(1):52–70
Neese F (2003) Metal and ligand hyperfine couplings in transition metal complexes: the effect of spin-orbit coupling as studied by coupled perturbed Kohn-Sham theory. J Chem Phys 118(9):3939–3948
Neese F (2008) ORCA—an ab initio, density functional and semiempirical program package, 2.6 revision 63 edn. Universität Bonn, Bonn
Neese F, Solomon EI (1998) Calculation of zero-field splittings, g-values, and the relativistic nephelauxetic effect in transition metal complexes. Application to high-spin ferric complexes. Inorg Chem 37(26):6568–6582
Norris JR, Budil DE, Gast P, Chang CH, El-kabbani O, Schiffer M (1989) Correlation of paramagnetic states and molecular-structure in bacterial photosynthetic reaction centers—the symmetry of the primary electron-donor in Rhodopseudomonas viridis and Rhodobacter sphaeroides R-26. Proc Natl Acad Sci USA 86(12):4335–4339
Parr RG (1983) Density functional theory. Annu Rev Phys Chem 34:631–656. doi:10.1146/annurev.pc.34.100183.003215
Plato M, Lendzian F, Lubitz W, Tränkle E, Möbius K (1988a) Molecular orbital studies on the primary donor P960 in reaction centers of Rps. viridis. In: Breton J, Vermeglio A (eds) The photosynthetic bacterial reaction center—structure and dynamics. Plenum Press, New York, pp 379–388
Plato M, Möbius K, Michel-Beyerle ME, Bixon M, Jortner J (1988b) Intermolecular electronic interactions in the primary charge separation in bacterial photosynthesis. J Am Chem Soc 110(22):7279–7285
Plato M, Möbius K, Lubitz W, Allen JP, Feher G (1990) Magnetic resonance and molecular orbital studies of the primary donor states in bacterial reaction centers. In: Jortner J, Pullman B (eds) Perspectives in photosynthesis. Kluwer, Dordrecht, pp 423–434
Pokkuluri PR, Laible PD, Deng YL, Wong TN, Hanson DK, Schiffer M (2002) The structure of a mutant photosynthetic reaction center shows unexpected changes in main chain orientations and quinone position. Biochemistry 41(19):5998–6007. doi:10.1021/bi0118963
Roszak AW, McKendrick K, Gardiner AT, Mitchell IA, Isaacs NW, Cogdell RJ, Hashimoto H, Frank HA (2004) Protein regulation of carotenoid binding: gatekeeper and locking amino acid residues in reaction centers of Rhodobacter sphaeroides. Structure 12(5):765–773
Roszak AW, Moulisova V, Reksodipuro ADP, Gardiner AT, Fujii R, Hashimoto H, Isaacs NW, Cogdell RJ (2012) New insights into the structure of the reaction centre from Blastochloris viridis: evolution in the laboratory. Biochem J 442:27–37. doi:10.1042/bj20111540
Schafer A, Horn H, Ahlrichs R (1992) Fully optimized contracted gaussian-basis sets for atoms Li to Kr. J Chem Phys 97(4):2571–2577
Schweiger A, Jeschke G (2001) Principles of pulse electron paramagnetic resonance. Oxford University Press, Oxford
Szabo A, Ostlund NS (1996) Modern quantum chemistry. Duver Publications, Inc., New York
Thurnauer MC (1979) ESR study of the photoexcited triplet state in photosynthetic bacteria. RevChem Intermed 100(3):197–231
Thurnauer MC, Norris JR (1977) Ordering of zero-field triplet spin sub-levels in chlorophylls—magneto-photoselection study. Chem Phys Lett 47(1):100–105
Thurnauer MC, Katz JJ, Norris JR (1975) Triplet-state in bacterial photosynthesis—possible mechanisms of primary photo-act. Proc Natl Acad Sci USA 72(9):3270–3274
Trosper TL, Benson DL, Thornber JP (1977) Isolation and spectral characteristics of photochemical reaction center of Rhodopseudomonas viridis. Biochim Biophys Acta 460(2):318–330
Volk M, Aumeier G, Langenbacher T, Feick R, Ogrodnik A, Michel-Beyerle ME (1998) Energetics and mechanism of primary charge separation in bacterial photosynthesis. A comparative study on reaction centers of Rhodobacter sphaeroides and Chloroflexus aurantiacus. J Phys Chem B 102(4):735–751
Weber S (2000) Recent EPR studies on the bacterial photosynthetic reaction center. In: Gilbert BC, Davies MJ, McLauchlan KA (eds) Electron paramagnetic resonance, vol 17. Royal Society of Chemistry, Cambridge, pp 43–77, and references therein
Weigend F, Haser M (1997) RI-MP2: first derivatives and global consistency. Theor Chem Acc 97(1–4):331–340
Yeates TO, Komiya H, Rees DC, Allen JP, Feher G (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26—membrane-protein interactions. Proc Natl Acad Sci USA 84(18):6438–6442
Yeates TO, Komiya H, Chirino A, Rees DC, Allen JP, Feher G (1988) Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1-protein-cofactor (bacteriochlorophyll, bacteriopheophytin, and carotenoid) interactions. Proc Natl Acad Sci USA 85(21):7993–7997
Acknowledgments
The authors thank Prof. Dr. K. Möbius for helpful discussions and G. Schmitz and K. Wrede (MPI CEC) for their help with the purification of reaction centers of Rb. sphaeroides R.26.1 and Bl. viridis wild type. H. Selbach and M. Reus (MPI CEC) are thanked for their help and kind advice at various stages of the preparations. This project was supported by the DFG-NWO international collaborative research grant project GA1100/1-2 and the MPG.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Full dataset of hyperfine couplings from the ENDOR spectra and DFT calculations. This material is available free of charge via the Internet at http://www.springerlink.com.
Rights and permissions
About this article
Cite this article
Marchanka, A., Lubitz, W., Plato, M. et al. Comparative ENDOR study at 34 GHz of the triplet state of the primary donor in bacterial reaction centers of Rb. sphaeroides and Bl. viridis . Photosynth Res 120, 99–111 (2014). https://doi.org/10.1007/s11120-012-9786-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9786-x