Abstract
Light energy harvested by the pigments in Photosystem I (PSI) is used for charge separation in the reaction center (RC), after which the positive charge resides on a special chlorophyll dimer called P700. In studies on the PSI trapping kinetics, P700+ is usually chemically reduced to re-open the RCs. So far, the information available about the reduction rate and possible chlorophyll fluorescence quenching effects of these reducing agents is limited. This information is indispensible to estimate the fraction of open RCs under known experimental conditions. Moreover, it would be important to understand if these reagents have a chlorophyll fluorescence quenching effects to avoid the introduction of exogenous singlet excitation quenching in the measurements. In this study, we investigated the effect of the commonly used reducing agent phenazine methosulfate (PMS) on the RC and fluorescence emission of higher plant PSI–LHCI. We measured the P700+ reduction rate for different PMS concentrations, and show that we can give a reliable estimation on the fraction of closed RCs based on these rates. The data show that PMS is quenching chlorophyll fluorescence emission. Finally, we determined that the fluorescence quantum yield of PSI with closed RCs is 4% higher than if the RCs are open.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosystem I (PSI) plays a major role in the light harvesting reaction of photosynthesis. The structure of the cyanobacterial PSI core complex has been solved at 2.5 Å resolution, it consists of at least 12 proteins, which coordinate 96 Chlorophylls (Chls) a, β-carotene, 2 phylloquinones, and 3 Fe4S4 clusters (Jordan et al. 2001). Higher plant PSI has a very similar structure as the complex of cyanobacteria (Ben-Shem et al. 2003), but in addition it contains four light harvesting antenna’s (Lhca) (Lam et al. 1984; Ben-Shem et al. 2003; Boekema et al. 2001). These Lhca’s bind carotenoids, Chls a and b and serve to increase the absorption cross section (Schmid et al. 1997; Croce et al. 2002). In green algae, the antenna system is even larger. The PSI complex of Chlamydomonas reinthardtii is believed to coordinate up to 14 Lhca antennae (Germano et al. 2002; Busch et al. 2010) which would mean that it can bind more than 300 Chls. In the higher plant PSI-LHCI structure, 173 Chls were assigned (Amunts et al. 2010).
Light energy harvested by this large number of pigments is efficiently transferred to the reaction center (RC), located in the core complex, where primary charge separation occurs. The common view is that a Chl a dimer called P700 is the primary electron donor, after charge separation the released electron is transferred along the electron transport chain: A0 (Chl a), A1 (phylloquinone), and the Fe4S4 clusters FX, FA, and FB, reviewed in Brettel (1997). Alternatively, it has been proposed that the accessory Chl(s), located in the proximity of P700, are instead the primary electron donor, while P700 only gets oxidized in the secondary electron transfer step (Holzwarth et al. 2006; Di Donato et al. 2011).
If PSI is in its natural environment, i.e., associated with the thylakoid membrane in cyanobacteria or chloroplasts, the electron from FB is donated to ferredoxin (or flavodoxin), while the hole on P700+ is filled by an electron coming from plastocyanin (or cytochrome c6). To understand the trapping kinetics of PSI, its excited state decay has been extensively investigated with different time-resolved techniques: transient absorption, emission measured by a synchroscan streak camera, time correlated single photon counting (TCSPC), or fluorescence up-conversion. Under generally applied experimental conditions, the endogenous oxidizing and reducing agents are not present. In absence of electron donors and acceptors, charge recombination occurs on the μs to ms time-scale, (e.g., Brettel 1997; Vassiliev et al. 1997). However, electrons can also escape from the Fe4S4 cluster to other electron acceptors, such as oxygen (Rousseau et al. 1993). Therefore, in absence of electron donors and presence of light all P700s are soon blocked in their oxidized (closed/P700+) state (Savikhin 2006). To study the kinetics of PSI with open RCs, reducing agents are added to the buffer. Most often phenazine methosulfate (PMS) reduced by sodium ascorbate (NaAsc) is used for this purpose. PMS is supplied at different concentrations: 10 μM (e.g., Gobets et al. 2001; Ihalainen et al. 2005; Turconi et al. 1993), 20 μM (Engelmann et al. 2006; Giera et al. 2010; Karapetyan et al. 1997; Nuijs et al. 1986), 60 μM (Slavov et al. 2008) or 150 μM (Byrdin et al. 2000).
In this work, we study how fast PMS re-reduces P700+ to its neutral state, and use these rates to estimate the fraction of closed RCs under different light intensities. We show that PMS itself is quenching fluorescence of light harvesting complexes. And we show that closing the RC of higher plant PSI increased the fluorescence quantum yield by only 4%.
Materials and methods
Purification of photosynthetic complexes
Thylakoids were isolated from Arabidopsis thaliana plants as described previously (Bassi and Simpson 1987). The major light harvesting complex of PSII (LHCII) and the PSI complex were obtained by mild solubilization of the thylakoids followed by the sucrose gradient density centrifugation, as described in (Caffarri et al. 2001). For all the fluorescence measurements, the obtained PSI complexes were run over a second sucrose gradient to improve the purity. Indeed, the low temperature emission shows that the sample is very pure (Wientjes et al. 2009). Photosystem II membranes were obtained as described in Berthold et al. (1981). The PSI light-harvesting antenna Lhca1/4 was obtained as described in Wientjes and Croce (2011).
Absorption and fluorescence spectroscopy
Absorption spectra were recorded on a Cary 4000 UV–Vis spectrophotometer (Varian, Palo Alto, CA). Fluorescence spectra were recorded on a Fluorolog 3.22 spectrofluorimeter (HORIBA Jobin-Yvon, Longjumeau, France); samples were diluted to an optical density of 0.05/cm at the Q y maximum, unless stated otherwise.
P700 and fluorescence kinetics
The P700 oxidative state and fluorescence kinetics were measured using the Dual-PAM-100 (Heinz Walz, Effeltrich, Germany). For P700+ detection, the 830 minus 875 nm absorption difference signal was used. The 635 nm actinic light was provided by a LED array (intensity as indicated). The fluorescence measuring light was operated at 40 μmol/m2/s with a frequency of 10 (in the PAM software), emission was detected through a RG9 filter (Schott). One ml of PSI solution was contained in a 1 × 1 × 3 cm cuvette, at an optical density of 3.3/cm in the Q y maximum.
All the measurements were performed at room temperature in 10 mM tricine, pH 7.8, 0.03% dodecyl-α-d-maltoside, and between 0 and 1 M sucrose.
Results
P700 reduction rate
We tested the P700 reduction rate for commonly used PMS/NaAsc concentrations on higher plant PSI. The broad 800–840 nm absorption band of oxidized P700 was employed to monitor the oxidation state during the reduction of P700 after a strong light pulse (Fig. 1). The traces were fitted with a mono-exponential decay function. The obtained reduction rate constants were 36, 204, and 412/s for 10, 60, and 150 μM PMS, respectively, with a standard deviation of ≤5% from four repetitions. The rates are similar to those reported previously for PSI of the cyanobacteria Synechocystis sp. PCC 6803 (Gourovskaya et al. 1997) and Synechococcus elongatus (Byrdin et al. 2000). If only 10 mM NaAsc was supplied as reducing agent, the rate constant was 0.053/s. This is six times faster than what is reported in Savikhin et al. (2001). The mono-exponential decay and the decay constant of ~20 s for NaAsc indicates that charge recombination, which takes place on the μs to ms time-scale, does not play a role in the P700+ reduction reported here.
Rate of photo-oxidized P700 reduction by PMS. The 830 minus 875 nm absorption signal is monitored after P700 is oxidized by a 20 mmol/m2/s light pulse with a duration of 0.2 s. PMS/NaAsc concentrations were as in previous reports: 10 μM/10 mM (e.g., Ihalainen et al. 2005), 60 μM/40 mM (Slavov et al. 2008), and 150 μM/5 mM (Byrdin et al. 2000)
Fraction of open RCs
For spectroscopic measurements on PSI, it is often claimed that the RCs are open before excitation. The fraction of open RCs can, in principle, be calculated based on the experimental conditions and the P700 reduction rate. To validate these theoretical calculations, we measured the fraction of closed RCs under a range of different light intensities and PMS concentrations. Figure 2 shows an example of these measurements, the P700+ concentration reaches 75% of the maximum during illumination with 531 μmol/m2/s of light if 10 μM PMS is supplied, while it reaches only 14% for 150 μM. For the maximum of P700+, the concentration reached under the strong light pulse of the 10 μM PMS data was used, because the fast reduction rate of 150 μM PMS does not allow to close all the reaction centers even if 20 mmol/m2/s of light is used.
P700+ build-up for different PMS concentrations. The P700+ formation upon illumination of PSI using 531 μmol/m2/s of actinic light (gray bar) was compared with that after a strong light pulse of 20 mmol/m2/s (white bar), the rest of the time the light was off (black bar) to allow for full re-opening of the RCs. PMS was reduced using NaAsc, at concentrations reported in the legend of Fig. 1
The combination of the charge separation and P700+ reduction rates determine the fraction of closed RCs in equilibrium, see Equation box 1. The charge separation rate depends mainly on the number of absorbed photons per PSI per second, which can be calculated if the excitation conditions are known. In the experiment described above, 531 μmol/m2/s of light was used and the excitation area was 1 cm2, thus 5.31 × 10−8 mol photons/s are fired at the sample. The optical density was 0.85/cm at the excitation wavelength (635 nm), with a cuvette path length of 1 cm this means that 10−0.85 is 14% of the light is transmitted, thus the absorptance is 86%, meaning that 4.56 × 10−8 mol photons/s are absorbed by PSI. We estimated that the extinction coefficient of Chl a and b is approximately the same at 635 nm and around 14000/M/cm, with ~170 Chls per higher plant PSI complex (Amunts et al. 2010) this gives an extinction coefficient of 2.38 × 106/M/cm for PSI. This means that in the measured volume of one cubic centimeter (10−3 l), the number of PSI complexes is 0.85/2.38 × 106/103 is 3.57 × 10−10 mol. Thus, on average each PSI absorbs 4.56 × 10−8/3.57 × 10−10 is 128 photon/s. We assume that PSI operates with an efficiency of close to 100%, thus roughly each absorbed photon results in charge separation. With a P700 reduction rate of 36/s as found in presence of 10 μM PMS, this means that k f /(k f + k b ) = 128/(36 + 128) = 78% of the RCs is expected to be closed (Equation box 1), while for a reduction rate of 412/s (150 μM PMS) 24% of the RCs is expected to be closed.
Figure 3 shows the calculated fraction of closed RCs against the measured values. The almost perfect correlation for the 10 PMS data points show that the calculation indeed gives meaningful information. For 60 μM PMS, the measured fraction of closed RCs is somewhat lower than the calculated one, while this difference is more pronounced for 150 μM PMS. These differences can be explained by the actual PSI efficiency being smaller than ~100%. A considerable lower efficiency is indeed expected for the 60 and 150 μM data, because PMS is a strong Chls fluorescence quencher (see below).
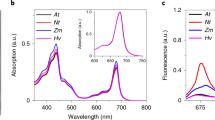
PMS is a fluorescence quencher
To avoid the introduction of artifacts in the measurements the reducing agent used to re-open the PSI RC should not by itself have an effect on the fluorescence. To investigate whether this is the case for PMS, we added it to a LHCII solution. Figure 4 shows the result. Addition of oxidized PMS did not affect the fluorescence intensity; however, as soon as it was reduced by NaAsc the intensity rapidly dropped. This effect was independent of the light intensity used. NaAsc itself did not reduce the fluorescence yield. Adding NaAsc first followed by PMS initially gave a similar result; however, for the higher PMS concentrations the solution rapidly became turbid. This turbidity was also observed in absence of Lhc complexes, and can possibly be explained by the aggregation of PMS. The addition of PMS followed by NaAsc reduced the fluorescence intensity by a factor of 2 for 10 μM, 18 for 60 μM, up to a factor of 64 for the highest concentration. The absorption of reduced PMS at these concentrations is below 0.05/cm for wavelengths longer than 500 nm, thus direct absorption of either excitation or emission light by PMS cannot explain the results. Therefore, it has to be concluded that PMS is quenching the chlorophyll emission. To investigate whether this is a general property, 60 μM of PMS and 40 mM of NaAsc were also added to PSII membranes (BBY’s, Berthold et al. 1981) and the PSI antenna complex Lhca1/4. In both the cases, the fluorescence was strongly quenched, by 11 and 15 times, respectively. We also tested whether N,N,N’,N’-tetramethyl-p-phenylenediamine (TMPD) is also quenching the LHCII emission. This is another reducing agent, which we found capable of reducing P700+ with a rate of 33/s at 2 mM concentration. Unfortunately, also TMPD was found to quench the LHCII emission.
Fluorescence emission of LHCII and PSI followed in time during the addition of PMS and NaAsc. For LHCII, the excitation was at 630 nm and the emission was detected at 680 nm; for PSI, the excitation was at 500 nm and the emission was detected at 725 nm. Excitation of PSI at 630 nm gave similar results
We proceeded to investigate the effect of PMS on the emission of PSI. Addition of 10 μM reduced PMS decreased the fluorescence intensity by 23%. Based on the excitation power of ~250 μmol/m2/s (at 500 nm), the 1.5 times larger PSI extinction coefficient at 500 nm compared with 635 nm, and the reduction rate of 36/s. It was estimated as described above that ~80% of the RCs were still closed under these conditions, indicating that the drop in fluorescence is due to the PMS quenching effect and not due to the different trapping efficiencies of P700 versus P700+. The quenching effect was more pronounced for the higher PMS concentrations. The emission intensity dropped more than three times. The combination of 150 μM PMS and 5 mM NaAsc, by itself, also showed some emission under the measuring conditions, meaning that the actual quenching was even greater. For the combination of 60 μM PMS and 40 mM NaAsc, we tested whether the extent of quenching was dependent on the PSI concentration. Increasing the PSI concentration six times, did not alter the level of PMS quenching, thus indicating that the level of quenching is only dependent on the PMS and not on the PSI concentration. Addition of NaAsc alone (40 mM) did not affect the fluorescence intensity.
Closing of PSI RCs slightly increases the fluorescence quantum yield
The need for re-reducing the RCs when studying the PSI trapping efficiency is not completely obvious as the overall trapping lifetime of PSI with open or closed RCs is usually found to be very similar (Savikhin et al. 2000; Nuijs et al. 1986; Owens et al. 1988; Turconi et al. 1993), although for the cyanobacterium Synechococcus elongatus a notable difference of 10% has been found (Byrdin et al. 2000). To get quantitative data on higher plant PSI we investigated the change in the fluorescence quantum yield (and thus in the trapping efficiency) upon closing the RCs of higher plant PSI. The possibility, of the Dual-PAM-100, to simultaneously detect the P700 oxidation state and the chlorophyll fluorescence, was used. The fluorescence signal is recorded by a pulse modulated measuring light which is operated at a low frequency. This allows us to record the PSI emission while most of the RCs remain open. The fluorescence excited by the much stronger actinic or saturating light is not detected. In our experiment, the fluorescence measuring light closed approximately 5% of the RCs (Fig. 5). Switching on the actinic light closed >95% of the RCs. This resulted on average (from 15 repetitions) in a 3.6% increase of the fluorescence emission, as this is caused by closing of >90% of the RCs this means that closing of all the RCs increases the fluorescence emission by 4% (with a standard deviation of 0.7%). It is noted that the increase/decrease of PSI emission in the light/dark follows the P700+ reduction kinetics, thus showing that the P700 oxidative state is indeed responsible for the change of the fluorescence quantum yield.
Simultaneous detection of fluorescence emission and P700+ absorption of PSI. The fluorescence emission of PSI was followed during the photo-oxidation of P700 using 70 μmol/m2/s of actinic light (gray bar) and the re-opening of the RCs in the dark by 10 mM NaAsc (black bar). The maximum level of P700+ was determined by a 500 ms saturating light pulse of 8000 μmol/m2/s (white bar)
Discussion
Although in virtually all the studies investigating the PSI trapping kinetics reducing agents are added to re-open the RCs, there are very few reports on the effect of these reagents. To our knowledge, there is just one study of the P700 reduction rate as function of the PMS concentration (Gourovskaya et al. 1997), while Byrdin et al. (2000) reported the reduction rate for the specific concentration used in their work. Further, we found one comment by Bulychev and Vredenberg (2001) that PMS at concentrations ≥5 μM is a light-dependent quencher for chlorophyll fluorescence of thylakoids. In this study, we investigated (i) the P700+ reduction rate in the presence of different PMS concentrations in order to estimate (i) which fraction of RCs is closed at specific light intensities, (ii) the chlorophyll fluorescence quenching effect of PMS, and (iii) the difference in fluorescence quantum yield of PSI with open and closed RCs.
RC: open or closed?
Although in most of the spectroscopic PSI experiments reported in the literature, it is claimed that the RCs are open, quantitative data are usually not presented. In a typical synchroscan streak-camera experiment on PSI the excitation light intensity is ~100 μW, the repetition rate is 150 kHz, the path lengths is 2 mm, and the spot diameter is 150 μm ( e.g., Ihalainen et al. 2005). Taking into account the photon energy of the excitation wavelength the number of photons per second and per pulse can be obtained. And based on the absorption of the sample, the estimated extinction coefficient and the excited volume, the number of photons absorbed per PSI per second and per pulse can be calculated. In the Result section, we have shown that this information can be employed to give a reliable estimation of the fraction of closed RCs. In the experiment of Ihalainen et al. (2005), the number of photons absorbed per PSI per pulse was ~0.3, thus 45000 photons/PSI/s. With a P700 reduction rate of 36/s (using 10 μM of PMS), 99.9% of the RCs would be closed. To lower the excitation pressure, the sample was contained in a spinning cuvette with a diameter of 10 cm and rotated at a speed of 30 Hz. As there is space for ~2000 spots on the circle, the average number of absorbed photons/PSI/s/spot is lowered to 23. However, taking into account the reduction rate of 36/s, still ~40% of the RCs are expected to be closed. This number is probably even higher because the sample is hit by 2.4 pulses while passing through the excitation spot, meaning that there is a large probability to hit one PSI twice during the short passes time.
One solution to lower the fraction of closed RCs, under very similar experimental conditions, is to increase the PMS concentration, ( see e.g., Giera et al. 2010). However, this will also increase the PMS chlorophyll fluorescence quenching (Fig. 4). A more elegant way to keep the RCs open is given by Müller et al. (2003). They use a spinning cuvette, which also moves sideways, in this system the excitation cycle time of the same volume is ~1 min (Müller et al. 2003). With a rotation speed of 70 Hz (Slavov et al. 2008), this would allow to have ~100% of the RCs open in the streak-camera experiment described above, even if PSI was reduced at a rate of 4/s. Such a reduction rate can be obtained using 1 μM of PMS, which will not notably quench the fluorescence (Bulychev and Vredenberg 2001). The special spinning cuvette also allows performing transient absorption (Müller et al. 2003; Holzwarth et al. 2006) and TCSPC (Slavov et al. 2008) experiments with nearly all the PSI RCs in open state.
Another obvious solution to lower the fraction of closed RCs is to lower the excitation power. For a very sensitive technique, for example TCSPC, this can still give data with a good signal to noise ratio. However, for the other techniques such as fluorescence up-conversion, this will not be possible, and one might have to settle with measuring PSI with closed RCs (Kennis et al. 2001).
PMS: to add or not to add?
Our study shows that the commonly used reducing agent PMS quenches the fluorescence emission of PSI. This effect might be avoided using very low concentrations of PMS (Bulychev and Vredenberg 2001), but under this condition the P700 reduction rate is also low. Another disadvantage of PMS is its low stability in water. Decomposition of solutions in deionized water takes only hours, while the stability is even lower in neutral buffers (Sigma Product Information sheet). Thus, during long measurements the actual PMS concentration, and thus the P700 reduction rate, will be lower than expected. The best solution would be to find a stable and fast P700 reducing agent that does not quench chlorophyll fluorescence. In the absence of such a reagent it can be preferable, depending on the goal of the experiment, to measure PSI with closed RCs as the fluorescence quantum yield and thus the trapping efficiency is only slightly dependent on the P700 oxidative state (Fig. 5).
Abbreviations
- PSI:
-
Photosystem I
- RC:
-
Reaction center
- PMS:
-
Phenazine methosulfate
- Chl:
-
Chlorophyll
- Lhca:
-
Light harvesting antennas of PSI
- TCSPC:
-
Time correlated single photon counting
- NaAsc:
-
Sodium ascorbate
- LHCII:
-
The major light harvesting complex of Photosystem II
- TMPD:
-
N,N,N’,N’-tetramethyl-p-phenylenediamine
References
Amunts A, Toporik H, Borovikova A, Nelson N (2010) Structure determination and improved model of plant photosystem I. J Biol Chem 285:3478–3486
Bassi R, Simpson D (1987) Chlorophyll-protein complexes of barley photosystem-I. Eur J Biochem 163:221–230
Ben-Shem A, Frolow F, Nelson N (2003) Crystal structure of plant photosystem I. Nature 426:630–635
Berthold DA, Babcock GT, Yocum CF (1981) A highly resolved, oxygen-evolving photosystem-II preparation from spinach thylakoid membranes—electron-paramagnetic-res and electron-transport properties. FEBS Lett 134:231–234
Boekema EJ, Jensen PE, Schlodder E, van Breemen JFL, van Roon H, Scheller HV, Dekker JP (2001) Green plant photosystem I binds light-harvesting complex I on one side of the complex. Biochemistry 40:1029–1036
Brettel K (1997) Electron transfer and arrangement of the redox cofactors in photosystem I. Biochim Biophys Acta 1318:322–373
Bulychev AA, Vredenberg WJ (2001) Modulation of photosystem II chlorophyll fluorescence by electrogenic events generated by photosystem I. Bioelectrochemistry 54:157–168
Busch A, Nield J, Hippler M (2010) The composition and structure of photosystem I-associated antenna from Cyanidioschyzon merolae. Plant J 62:886–897
Byrdin M, Rimke I, Schlodder E, Stehlik D, Roelofs TA (2000) Decay kinetics and quantum yields of fluorescence in photosystem I from Synechococcus elongatus with P700 in the reduced and oxidized state: are the kinetics of excited state decay trap-limited or transfer-limited? Biophys J 79:992–1007
Caffarri S, Croce R, Breton J, Bassi R (2001) The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J Biol Chem 276:35924–35933
Croce R, Morosinotto T, Castelletti S, Breton J, Bassi R (2002) The Lhca antenna complexes of higher plants photosystem I. Bba-Bioenergetics 1556:29–40
Di Donato M, Stahl AD, van Stokkum IHM, van Grondelle R, Groot ML (2011) Cofactors Involved in light-driven charge separation in photosystem I identified by subpicosecond infrared spectroscopy. Biochemistry 50:480–490
Engelmann E, Zucchelli G, Casazza AP, Brogioli D, Garlaschi FM, Jennings RC (2006) Influence of the photosystem I–light harvesting complex I antenna domains on fluorescence decay. Biochemistry 45:6947–6955
Germano M, Yakushevska AE, Keegstra W, van Gorkom HJ, Dekker JP, Boekema EJ (2002) Supramolecular organization of photosystem I and light-harvesting complex I in Chlamydomonas reinhardtii. FEBS Lett 525:121–125
Giera W, Ramesh VM, Webber AN, van Stokkum I, van Grondelle R, Gibasiewicz K (2010) Effect of the P700 pre-oxidation and point mutations near A0 on the reversibility of the primary charge separation in photosystem I from Chlamydomonas reinhardtii. Biochim Biophys Acta 1797:106–112
Gobets B, van Stokkum IHM, Rogner M, Kruip J, Schlodder E, Karapetyan NV, Dekker JP, van Grondelle R (2001) Time-resolved fluorescence emission measurements of photosystem I particles of various cyanobacteria: a unified compartmental model. Biophys J 81:407–424
Gourovskaya KN, Mamedov MD, Vassiliev IR, Golbeck JH, Semenov AY (1997) Electrogenic reduction of the primary electron donor P700(+) in photosystem I by redox dyes. FEBS Lett 414:193–196
Holzwarth AR, Müller MG, Niklas J, Lubitz W (2006) Ultrafast transient absorption studies on photosystem I reaction centers from Chlamydomonas reinhardtii. 2: mutations near the P700 reaction center chlorophylls provide new insight into the nature of the primary electron donor. Biophys J 90:552–565
Ihalainen JA, van Stokkum IHM, Gibasiewicz K, Germano M, van Grondelle R, Dekker JP (2005) Kinetics of excitation trapping in intact photosystem I of Chlamydomonas reinhardtii and Arabidopsis thaliana. Biochim Biophys Acta 1706:267–275
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 angstrom resolution. Nature 411:909–917
Karapetyan NV, Dorra D, Schweitzer G, Bezsmertnaya IN, Holzwarth AR (1997) Fluorescence spectroscopy of the longwave chlorophylls in trimeric and monomeric photosystem I core complexes from the cyanobacterium Spirulina platensis. Biochemistry 36:13830–13837
Kennis JTM, Gobets B, van Stokkum IHM, Dekker JP, van Grondelle R, Fleming GR (2001) Light harvesting by chlorophylls and carotenoids in the photosystem I core complex of Synechococcus elongatus: a fluorescence upconversion study. J Phys Chem B 105:4485–4494
Lam E, Oritz W, Mayfield S, Malkin R (1984) Isolation and characterization of a light-harvesting chlorophyll a/B protein complex associated with photosystem-I. Plant Physiol 74:650–655
Müller MG, Niklas J, Lubitz W, Holzwarth AR (2003) Ultrafast transient absorption studies on photosystem I reaction centers from Chlamydomonas reinhardtii. 1. A new interpretation of the energy trapping and early electron transfer steps in photosystem I. Biophys J 85:3899–3922
Nuijs AM, Shuvalov VA, Vangorkom HJ, Plijter JJ, Duysens LNM (1986) Picosecond absorbency difference spectroscopy on the primary reactions and the antenna-excited states in photosystem-I particles. Biochim Biophys Acta 850:310–318
Owens TG, Webb SP, Alberte RS, Mets L, Fleming GR (1988) Antenna structure and excitation dynamics in photosystem-I. 1. Studies of detergent-isolated photosystem-I preparations using time-resolved fluorescence analysis. Biophys J 53:733–745
Rousseau F, Setif P, Lagoutte B (1993) Evidence for the involvement of Psi-E subunit in the reduction of ferredoxin by photosystem-I. EMBO J 12:1755–1765
Savikhin S (2006) Ultrafast optical spectroscopy of photosystem I. In: Golbeck J (ed) Photosystem I: the light-driven plastocyanin : ferredoxin oxidoreductase, vol 24. Springer, Dordrecht, pp 155–175
Savikhin S, Xu W, Chitnis PR, Struve WS (2000) Ultrafast primary processes in PS I from Synechocystis so. PCC 6803: roles of P700 and A(o). Biophys J 79:1573–1586
Savikhin S, Xu W, Martinsson P, Chitnis PR, Struve WS (2001) Kinetics of charge separation and A(0)(−)-> A(1) electron transfer in photosystem reaction centers. Biochemistry 40:9282–9290
Schmid VHR, Cammarata KV, Bruns BU, Schmidt GW (1997) In vitro reconstitution of the photosystem I light-harvesting complex LHCI-730: Heterodimerization is required for antenna pigment organization. Proc Natl Acad Sci USA 94:7667–7672
Slavov C, Ballottari M, Morosinotto T, Bassi R, Holzwarth AR (2008) Trap-limited charge separation kinetics in higher plant photosystem I complexes. Biophys J 94:3601–3612
Turconi S, Schweitzer G, Holzwarth AR (1993) Temperature-dependence of picosecond fluorescence kinetics of a cyanobacterial photosystem-I particle. Photochem Photobiol 57:113–119
Vassiliev IR, Jung YS, Mamedov MD, Semenov AY, Golbeck JH (1997) Near-IR absorbance changes and electrogenic reactions in the microsecond-to-second time domain in photosystem I. Biophys J 72:301–315
Wientjes E, Croce R (2011) The light-harvesting complexes of higher plant photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem J 433:477–485
Wientjes E, Oostergetel GT, Jansson S, Boekema EJ, Croce R (2009) The Role of Lhca complexes in the supramolecular organization of higher plant photosystem I. J Biol Chem 284:7803–7810
Acknowledgments
This study was supported by the De Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO), Earth and Life Sciences (ALW), through a Vidi grant (to R.C.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wientjes, E., Croce, R. PMS: Photosystem I electron donor or fluorescence quencher. Photosynth Res 111, 185–191 (2012). https://doi.org/10.1007/s11120-011-9671-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-011-9671-z