Abstract
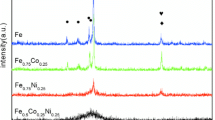
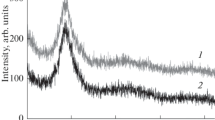
Thin ribbons of the metallic glass Mg65Cu25Y10 and Mg63Ni30Y7 obtained by melt spinning were saturated with atomic hydrogen during electrochemical decomposition of water. The amount of absorbed hydrogen was as high as 4 mass% for the alloy Mg65Cu25Y10Hx and 1.5 mass% for the alloy Mg63Ni30Y7Hx. As the hydrogen content increases up to 3.6 mass%, the amorphous structure of the copper-containing alloy is transformed to a nanocrystalline structure with formation of magnesium and yttrium hydrides and also the intermetallic Cu2Mg at room temperature. The appearance of crystalline compounds during saturation with hydrogen leads to a decrease in the thermal stability of the amorphous alloy and a shift of the differential scanning calorimetry curves toward lower temperatures. In the presence of nickel, the thermal stability of the amorphous alloy Mg63Ni30Y7Hx increases with hydrogen saturation. After heating up to the crystallization temperature, the compounds Mg2NiH0.3 and YH2 appear.
Similar content being viewed by others
References
A. Gebert, U. Wolff, M. Savyak, et al., “Stability and electrochemical properties of Mg65Cu25Y10 metallic glass,” Mater. Sci. Forum, 377, 9–14 (2001).
M. Savyak, S. Hirny, H.-D. Bauer, et al., “Electrochemical hydrogenation of Mg65Cu25Y10, ” J. Alloys and Comp., 364, 217–228 (2003).
M. P. Savyak, A. Gebert, and M. Uhlemann, “Effect of hydrogen on the amorphous structure of Mg65Cu25Y10 alloy with electrochemical saturation,” Poroshk. Metall., Nos. 9–10, 1–7 (2004).
A. Gebert, U. Wolff, A. John, et al., “Stability of the bulk glass-forming Mg65Cu25Y10 alloy in aqueous electrolytes,” Mater. Sci. Eng., A299, 125–135 (2001).
A. Gebert, K. Buchholz, A. Leohard, et al., “Investigations on the electrochemical behaviour of Zr-based bulk metallic glasses,” Mater. Sci. Eng., A267, No. 2, 294–300 (1999).
A. Zaluska, L. Zaluski, and J. O. Strom-Olsen, “Nanocrystalline magnesium for hydrogen storage, ” J. All. Comp., 288, 217–225 (1999).
J. Eckert, “Massive amorphous metals,” Wissenschaftliche Zeitschrift der Technischen Universität Dresden, 46, No. 3, 86–90 (1997).
A. Inoue and T. Masumoto, “Mg-based amorphous alloys,” Mater. Sci. Eng., A173, 1 (1993).
T. Spassov and U. Köster, “Thermal stability and hydriding properties of nanocrystalline melt-spun Mg63Ni30Y7,” J. All. Comp., 279, 279–286 (1998).
T. Spassov, P. Solsona, S. Surinach, and M. D. Baro, “Nanocrystallization in Mg83Ni17−x Yx (x = 0, 7.5) amorphous alloys,” J. All. Comp., 345, 123–129 (2002).
N. Ismail, M. Uhlemann, A. Gebert, and J. Eckert, “Hydrogenation and its effect on the crystallisation behaviour of Zr55Cu30Al10Ni5 metallic glass,” J. All. Comp., 298, 146 (2000).
I. G. Konstanchuk, E. Yu. Ivanov, and V. V. Boldyrev, “Reaction with hydrogen of alloys and intermetallics obtained by mechanochemical methods,” Usp. Khim., 67, 7–75 (1998).
Author information
Authors and Affiliations
Additional information
__________
Translated from Poroshkovaya Metallurgiya, Nos. 3–4(448), pp. 105–111, March–April, 2006.
Rights and permissions
About this article
Cite this article
Savyak, M.P. Thermal stability of amorphous alloys Mg65Cu25Y10, Mg63Ni30Y7 after electrochemical hydrogen absorption. Powder Metall Met Ceram 45, 196–201 (2006). https://doi.org/10.1007/s11106-006-0063-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11106-006-0063-4