Abstract
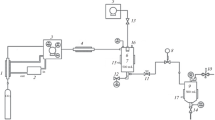
The effect of wet chemical synthesis parameters on the properties of nano-powders of zirconium dioxide stabilized with yttrium is studied. Features of nano-powder synthesis by the oxalate, hydroxide and thermal hydrolysis of a sol methods are determined. Nano-size zirconium dioxide powder stabilized with 3 mole% yttrium is prepared by hydrothermal coprecipitation from a sol of metal chlorides and urea followed by dispersion and calcination. The possibility of controlling particle morphology by changing synthesis conditions, subsequent treatment and ultrasonic dispersion of the powder, and the calcination temperature-time schedule are studied by experiment. A method is developed for optimizing particle morphology (level of aggregation (agglomeration capacity)) and size. Non-agglomerated zirconium dioxide powder consisting of uniform nanosized (about 45 nm) aggregates of primary crystals is synthesized.
Similar content being viewed by others
REFERENCES
F. F. Lange, “Powder processing science and technology for increased reliability,” J. Amer. Ceram. Soc., 72, No.1, 3–15 (1989).
W. H. Rhodes, “Agglomerate and particle size effect on sintering yttrium-stabilized zirconia,” J. Amer. Ceram. Soc., 64, No.1, 19–22 (1981).
M. Van de Graaf, J. Ter Maat, and A. Burggraaf, “Microstructure and sintering kinetics of highly reactive ZrO2-Y2O3 ceramics,” J. Mater. Sci., 20, 1407–1418 (1985).
S. Theunissen, A. Winnubst, and A. Burggraaf, “Sintering kinetics and microstructure development of nano-scale Y-TZP ceramics,” J. Eur. Ceram. Soc. 11, 315–324 (1993).
P. Duran, M. Villegas, F. Capel, and C. Mure, “Low temperature fully densified nanostructured Y-TZP ceramic,” J. Mater. Sci, 15, 741–744 (1996).
S. Lawsen, “Environment degradation of zirconia ceramics,” J. Eur. Ceram. Soc. 15, 485–502 (1995).
J. Lin and J. Duh, “Coprecipitation and hydrothermal synthesis of ultrafine 5.5 mole% CeO2-2 mole% YO1.5-ZrO2 powders,” J. Amer. Ceram. Soc., 80, No.1, 92–98 (1997).
H. Nishizawa, Y. Yamasaki, and K. Matsuoka, “Crystallization and transformation of zirconia under hydrothermal conditions,” J. Amer. Ceram. Soc., 65, No.7, 343–346 (1982).
E. Tani, M. Yoshimura, and S. Somiya, “Formation of ultrafine tetragonal ZrO2 powder under hydrothermal conditions,” J. Amer. Ceram. Soc., 66, No.1, 11–14 (1983).
J. Lin and J. Duh, “Crystallite size and microstrain of thermally aged low-ceria and low-yttrium-doped zirconia,” J. Amer. Ceram. Soc., 81, No.4, 853–860 (1998).
W. Luan, L. Gao, and J. Guo, “Study of drying stage of nano-scale powder preparation,” Nanostructured Mat., 10, No.7, 1119–1125 (1998).
N. Enomoto, S. Muruyama, and Z. Nakagawa, “Agglomeration of silicon spheres under ultrasonication,” J. Mater. Sci., 12, No.5, 1410–1415 (1997).
S. Kwon and G. L. Messing, “The effect of particle solubility on the strength of nanocrystalline agglomerates: boehmite,” Nanostructured Mat., 8, No.4, 399–418 (1997).
M. Kitayama and J. A. Pak, “Formation and control of agglomerates in alumina powder,” J. Amer. Ceram. Soc., 79, No.8, 2003–2011 (1996).
A. Maskara and D. M. Smith, “Agglomeration during of fine silica powders. Part. 2. The role of particle solubility,” J. Amer. Ceram. Soc., 80, No.7, 1715–1722 (1997).
A. Singhal, G. Skandan, A. Wang, et al., “Nanoparticle aggregation during vapor phase synthesis,” Nanostructured Mat., 11, No.4, 545–552 (1999).
M. Readey and D. Readey, “Sintering of ZrO2 in HCl atmospheres,” J. Amer. Ceram. Soc., 69, No.7, 580–582 (1986).
M. S. Kalishewski and A. H Heuer, “Alcohol interaction with zirconia powders,” J. Amer. Ceram. Soc., 73, No.6, 1504–1509 (1990).
R. Peterson and E. B. Slamovich, “Effect of processing parameters on the morphology of hydrothermally derived PbTiO3 powders,” J. Amer. Ceram. Soc., 82, No.7, 1702–1710 (1999).
E. Jorge, T. Chartier, and P. Boch, “Ultrasonic dispersion of ceramic powder,” J. Amer. Ceram. Soc., 73, No.8, 2552–2554 (1990).
T. Suzuki, Y. Sakka, K. Nakano, and K. Hiraga, “Effect of ultrasonication on colloidal dispersion of Al2O3 and ZrO2 powders in pH controlled suspension,” Mat. Trans., 39, No.6, 689–692 (1998).
D. Schmid and L. Sbaizero, “Ultrasonic homogenization of equivolumetric Al2O3/ZrO2 suspensions,” J. Mater. Sci., 35, 1213–1217 (2000).
O. Vasylkiv and Y. Sakka, “Nonisothermal synthesis of yttria stabilized zirconia nano-powder through oxalate processing. I. Peculiarities of (Y — Zr) oxalate synthesis and its decomposition,” J. Amer. Ceram. Soc., 83, No.9, 2196–2202 (2000).
O. Vasylkiv, Y. Sakka, and H. Borodianska “Nonisothermal synthesis of yttria stabilized zirconia nano-powder through oxalate processing. II. Morphology manipulation,” J. Amer. Ceram. Soc., 84, No.11, 2484–2488 (2001).
O. Vasylkiv and Y. Sakka, “Synthesis and sintering of zirconia nano-powder by non-isothermal decomposition from hydroxide,” J. Ceram. Soc. Jap., 189, 500–50 (2001).
O. Vasylkiv and Y. Sakka, “Hydroxide synthesis, colloidal processing and sintering of nano-size 3Y-TZP powder,” Scr. Mater., 44, 2219–2223 (2001).
O. Vasylkiv, Y. Sakka, and K. Hiraga, “Chemical synthesis and sintering of zirconia-based nano-powders,” Ceram. Trans. Amer. Ceram. Soc., 112, 11–16 (2001).
O. Vasylkiv and Y. Sakka, “Hydrothermal synthesis of nano-size ZrO2 powder, its characterization and colloidal processing,” Stud. Surf. Sci. Catal., 32, 233–236 (2001).
O. Vasylkiv and Y. Sakka, “Synthesis and colloidal processing of zirconia, nano-powder,” J. Amer. Ceram. Soc., 84, No.11, 2489–2494 (2000).
O. Vasylkiv, Y. Sakka, and V. V. Skorokhod, “Low-temperature processing and mechanical properties of zirconia and zirconia-alumina nano-ceramics,” J. Amer. Ceram. Soc., 86, No.2, 299–304 (2003).
Author information
Authors and Affiliations
Additional information
__________
Translated from Poroshkovaya Metallurgiya, Nos. 5–6(443), pp. 28–42, May–June, 2005.
Rights and permissions
About this article
Cite this article
Vasylkiv, O.O., Sakka, Y. & Skorokhod, V.V. Features of Preparing Nano-Size Powders of Tetragonal Zirconium Dioxide Stabilized with Yttrium. Powder Metall Met Ceram 44, 228–239 (2005). https://doi.org/10.1007/s11106-005-0086-2
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11106-005-0086-2