Abstract
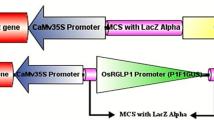
During germination, phytate, a major phosphate compound in seeds, is degraded into inorganic phosphorus (Pi) for differentiation of new tissues via catalysis. OsPHY1, a member of phytase genes in rice, has previously been found to show high expression levels in the endosperm and hypocotyl during germination and is involved in the degradation of seed phytate. In this study, we investigated the transcriptional mechanisms of OsPHY1 via OsPHY1 promoter-GUS analysis. The results of GUS histochemical staining and activities in tobaccos harboring OsPHY1-GUS reveal that the reporter gene is strongly expressed in the hypocotyl and responds to diverse stimuli cues initiated by phytohormones abscisic acid (ABA), gibberellin (GA3), and indole-3-acetic acid (IAA), as well as osmotic stresses of salt, drought, and cold. The results indicate that the cis-regulatory element CANBNNAPA regulates gene hypocotyl-predominant expression. ABRE and GAREAT are involved in gene responses to ABA and GA3, respectively, and DRECRTCOREAT is involved in gene responses to stresses of salt, drought, and cold. In addition, the responses of OsPHY1 and part-osmotic stress-responsive genes to salt, drought, and cold mediated by DRECRTCOREAT are largely accomplished through the ABA-dependent pathway with the involvement of the ABA responsive cis-regulatory element ABRE. Our results reveal that the degradation of seed phytate during germination mediated by OsPHY1 is regulated in a fine-tune manner with the involvement of diverse cis-regulatory elements at the transcription level.






Similar content being viewed by others
References
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Alvey L, Harberd NP (2005) DELLA proteins: integrators of multiple plant growth regulatory inputs? Physiol Plant 123:153–160
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103:1035–1040
Chen K, Fessehaie A, Arora R (2012) Selection of reference genes for normalizing gene expression during seed priming and germination using qPCR in Zea mays and Spinacia oleracea. Plant Mol Biol Rep 30:478–487
Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Dekkers BJW, Smeekens SCM (2007) Sugar and abscisic acid regulation of germination and transition to seedling growth. In: Bradford KJ, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell, Oxford, pp 305–327
Dionisio G, Madsen CK, Holm PB, Welinder KG, Jørgensen M, Stoger E, Arcalis E, Brinch-Pedersen H (2011) Cloning and characterization of purple acid phosphatase phytases from wheat, barley, maize, and rice. Plant Physiol 156:1087–1100
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33:751–763
Eeckhout W, De Paepe M (1994) Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim Feed Sci Technol 47:19–29
Ellerström M, Stalberg K, Ezcurra I, Rask L (1996) Functional dissection of a napin gene promoter: identification of promoter elements required for embryo and endosperm-specific transcription. Plant Mol Biol 32:1019–1027
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl):S15–S45
Gubler F, Hughes T, Waterhouse P, Jacobsen J (2008) Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiol 147:886–896
Guilfoyle T, Hagen G, Ulmasov T, Murfett J (1998) How does auxin turn on genes? Plant Physiol 118:341–347
Guo L, Zhao Y-X, Zhang S-H, Zhang H-N, Xiao K (2009) Improvement of organic phosphate acquisition in transgenic tobacco plants by overexpression of a soybean phytase gene Sphy1. Front Agric China 3:259–265
Hu B, Wan X, Liu X, Guo D, Li L (2010) Abscisic acid (ABA)-mediated inhibition of seed germination involves a positive feedback regulation of ABA biosynthesis in Arachis hypogaea L. Afr J Biotechnol 9:1578–1586
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Lei XG, Porres JM, Mullaney EJ, Brinch-Pedersen H (2007) Phytase: source, structure, and application. In: Polaina J, MacCabe AP (eds) Industrial Enzymes: Structure. Function and Applications. Springer, Dordrecht, The Netherlands, pp 505–530
Li R, Lu W, Gu J, Li X, Guo C, Xiao K (2011) Molecular characterization and functional analysis of OsPHY2, a phytase gene classified in histidine acid phosphatase type in rice (Oryza sativa L.). Afr J Biotechnol 10:11110–11123
Li R, Lu W, Guo C, Li X, Gu J, Xiao K (2012) Molecular characterization and functional analysis of OsPHY1, a purple acid phosphatase (PAP)-type phytase gene in rice (Oryza sativa L.). J Integr Agric 11(8):1217–1226
Linkies A, Gräber K, Knight C, Leubner-Metzger G (2010) The evolution of seeds. New Phytol 186:817–831
Loewus FA, Murthy PPN (2000) Myo-inositol metabolism in plants. Plant Sci 150:1–19
Lott JNA (1984) Accumulation of seed reserves of phosphorus and other minerals. In: Murray DR (ed) Seed Physiology. Academic Press, New York, pp 139–166
Mahnaz A, Fazli F, Bagherieh-Najjar MB (2012) Analyses of Arabidopsis trr14 T-DNA insertion mutants reveal an essential role in seed germination. Plant Mol Biol Rep 30:319–329
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Morris K, Linkies A, Müller K, Oracz K, Wang X, Lynn JR, Leubner-Metzger G, Finch-Savage WE (2011) Regulation of seed germination in the close Arabidopsis relative Lepidium sativum: a global tissue-specific transcript analysis. Plant Physiol 155:1851–1870
Mullaney EJ, Ullah AH (2003) The term phytase comprises several different classes of enzymes. Biochem Biophys Res Commun 312:179–184
Mullaney EJ, Daly CB, Ullah AH (2000) Advances in phytase research. Adv Appl Microbiol 47:157–199
Murata T, Akazawa T, Fukuchi S (1968) Enzymic mechanism of starch breakdown in germinating rice seeds. I. An analytical study. Plant Physiol 43:1899–1905
Murungu FS (2011) Effects of seed priming and water potential on seed germination and emergence of wheat (Triticum aestivum L.) varieties in laboratory assays and in the field. Afr J Biotechnol 10(21):4365–4371
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41:697–709
Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15:1591–1604
Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19:1192–1208
Palmiano EP, Juliano BO (1972) Biochemical changes in the rice grain during germination. Plant Physiol 49:751–756
Puhl AA, Gruninger RJ, Greiner R, Janzen TW, Mosimann SC, Selinger LB (2007) Kinetic and structural analysis of a bacterial protein tyrosine phosphatase-like myo-inositol polyphosphatase. Protein Sci 16(7):1368–1378
Riefler M, Novak O, Strnad M, Schmulling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48:354–366
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 503–512
Soeda Y, Konings MCJM, Vorst O, van Houwelingen AMML, Stoopen GM, Maliepaard CA, Kodde J, Bino RJ, Groot SPC, van der Geest AHM (2005) Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are indicators of progression of the germination process and the stress tolerance level. Plant Physiol 137:354–368
Szewińska J, Zdunek-Zastocka E, Pojmaj M, Bielawski W (2012) Molecular cloning and expression analysis of Triticale phytocystatins during development and germination of seeds. Plant Mol Biol Rep 30:867–877
Tamura N, Yoshida T, Tanaka A, Sasaki R, Bando A, Toh S, Lepiniec L, Kawakami N (2006) Isolation and characterization of high temperature-resistant germination mutants of Arabidopsis thaliana. Plant Cell Physiol 47:1081–1094
Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N et al (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146:1368–1385
Xiang Y, Tang N, Du H, Ye H, Xiong L (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148:1938–1952
Xiao K, Zhang C, Harrison M, Wang Z-Y (2005) Isolation and characterization of a novel plant promoter that directs strong constitutive expression of transgenes in plants. Mol Breed 15:221–231
Xu N, Hagen G, Guilfoyle TJ (1997) Multiple auxin response modules in the soybean SAUR 15A promoter. Plant Sci 126:193–201
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang C, Li A, Zhao Y, Zhan Z, Zhu Y, Tan X, Geng S, Guo H, Zhang X, Kang Z, Mao L (2011) Overexpression of a wheat CCaMK gene reduces ABA sensitivity of Arabidopsis thaliana during seed germination and seedling growth. Plant Mol Biol Rep 29:681–692
Yu S, Zhang F, Yu Y, Zhang D, Zhao X, Wang W (2012) Transcriptome profiling of dehydration stress in the Chinese cabbage (Brassica rapa L. ssp. pekinensis) by tag sequencing. Plant Mol Biol Rep 30:17–28
Zhou G, Yang L-T, Li Y-R, Zou C-L, Huang L-P, Qiu L-H, Huang X, Srivastava MK (2012) Proteomic analysis of osmotic stress-responsive proteins in sugarcane leaves. Plant Mol Biol Rep 30:349–359
Zhu JK, Hasegawa PM, Bressan RA (1997) Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci 16:253–277
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30871466), National Transgenic Major Program (No. 2011ZX08008) and Key Laboratory of Crop Growth Regulation of Hebei Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chengjin Guo, Li Guo and Xiaojuan Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, C., Guo, L., Li, X. et al. Transcriptional Regulation of the Rice Phytase Gene OsPHY1 by Several Phytohormones and Osmotic Stresses Using Promoter-GUS Analysis. Plant Mol Biol Rep 31, 1461–1473 (2013). https://doi.org/10.1007/s11105-013-0615-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-013-0615-y