Abstract
Aims
The alkaloid hordenine is one of the major allelochemicals involved in the allelopathic ability of barley (Hordeum vulgare L.), whose biosynthesis and accumulation is preferentially located in roots. Hordenine appears to have been unintentionally favored during domestication in modern and cultivated barley cultivars at the expense of another alkaloid, gramine. In this study, we assessed the content of hordenine and its two precursors, N-methyltyramine (NMT) and tyramine, in roots and root exudates of the modern spring barley cv. Solist, and particularly how they are affected due to nutrient deficiencies.
Methods
We monitored the three metabolites during the early phases of barley growth i.e., up to 8 days, applying HPLC time-course and both target and untargeted metabolomic approaches. Barley plants were grown either in full nutrient solutions or in specific nutrient shortage conditions (N, S, P and Fe).
Results
Results confirmed a strong decrease of the allelochemical accumulation (hordenine and the two precursors) in roots and in root exudates during both 24 h and 8 days time-course experiments. Yet, the overall tyramine content was approximately tenfold lower compared to the other two compounds. In addition, plants subjected to nitrogen (-N), sulfur (-S), phosphorus (-P) and iron (-Fe) deprivation showed nutrient-dependent accumulation of hordenine, N-methyltyramine and tyramine, as well as of other secondary metabolites. Indeed, the synthesis of hordenine and N-methyltyramine was trigged under nutrient deficiencies.
Conclusions
In conclusion, this study highlighted the impact of nutrient availability on the growth-dependent accumulation patterns of all the three compounds investigated in modern barley roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, allelopathic plant interactions have received growing attention by researchers, farmers and the international community as one of the most promising, effective and environmentally friendly strategies in weed management in crop fields (Farooq et al. 2011). It should be noticed that plant interactions and effects within the nearby environment belong to the phenomenon of allelopathy, defined by the International Allelopathy Society (IAS) as “any process involving secondary metabolites produced by plants, algae, microorganisms, viruses and fungi that influence the growth and the development of agricultural and biological systems” (IAS 1996). Plant secondary metabolites characterized by allelopathic properties are named allelochemicals (Whittaker and Feeny 1971). These compounds might be accumulated in either plant roots or leaves, or both. Furthermore, they can also be released into the environment by: i) volatilization, ii) leaching from aboveground plant tissues (mainly leaves), iii) root exudation and iv) decomposition of plant residues (Albuquerque et al. 2011). In addition, it has been demonstrated that the synthesis and release of these secondary metabolites might be further triggered when plants are subjected to abiotic or biotic stresses, e.g., high temperatures (Hanson et al. 1983) or pathogens infections (Matsuo et al. 2001).
Several commercial crops are well known for their natural ability to release allelochemicals and, thus, to compete with weeds. One of them is rice (Oryza sativa L.) (Kong et al. 2011), together with several other annual crop species like alfalfa (Medicago sativa L.), rye (Secale secale L.), wheat (Triticum aestivum L.), sunflower (Helianthus annus L.) and barley (Hordeum vulgare L.) (Jabran 2017). The latter is one of the first domesticated grains in human history and the fourth most important annual cereal crop in the world (www.fao.org). Moreover, barley has been known for centuries for its ability to suppress and inhibit weed growth and germination due to the production and release of allelochemicals (Kremer and Ben-Hammouda 2009). Among them, the two alkaloids gramine (N,N-dimethylindolemethylamine) and hordenine (N,N-dimethyltyramine) were proven as allelopathic alkaloids with phytotoxic effects for the first time in barley in 1966 (Overland 1966) and 1989 (Lovett et al. 1994), respectively. However, modern barley cultivars show a reduced allelopathic potential and allelochemicals accumulation as compared to wild relatives. This adaptation is likely to be an unintentional consequence of the domestication and selection processes, defined as “domestication syndrome” (Bertholdsson 2004; Oveisi et al. 2008; Vasilakoglou et al. 2009).
The alkaloid gramine has as precursor the amino acid tryptophan and is mainly accumulated in leaves of wild barley relatives. Indeed, in some modern cultivars the synthesis of gramine is extremely reduced, partially functioning (i.e., not complete biosynthetic pathway) or even completely lost like in cv. Barke and Morex (Larsson et al. 2006; Maver et al. 2020). On the other hand, much less is known about hordenine, even though its complete biosynthetic pathway has been identified, deriving from the amino acid tyrosine (Schenck and Maeda 2018) (Fig. 1). Besides its phytotoxic effects on susceptible weeds, hordenine is also involved in plant defense responses i) through the jasmonate-dependent defense pathway (Ishiai et al. 2016), ii) acting as an inhibitor of monoamine oxidase B (stimulator of gastrin) and iii) exhibiting antibacterial antibiotic properties on melanogenesis (Kim et al. 2013). It is interesting to note that, on the contrary of gramine, hordenine seems to be still accumulated in modern and cultivated barley with a preferential accumulation at root level (Maver et al. 2020). Furthermore, some evidence in literature suggests that hordenine biosynthesis has been unintentionally and/or indirectly favored during the domestication process at the expense of the other alkaloid gramine (Lovett and Hoult 1995; Belz 2007; Maver et al. 2020).
Hence, in order to better characterize the plant biosynthesis and accumulation of hordenine as well as its release in the growing medium in relation to the plant nutritional status, plants of spring modern barley cv. Solist were grown with different levels of nitrogen (N), sulfur (S), phosphorus (P) or iron (Fe) availability. These nutritional disorders have been chosen as they represent very common abiotic stresses in agricultural soils and are, therefore, likely to negatively or positively trigger/modulate the synthesis/accumulation dynamics of allelochemicals in barley. To this purpose, an approach based on detection and profiling of the alkaloid accumulation in plant tissues and exudates at intervals of up to 8 days, has been applied. The hordenine precursors N-methyltyramine (NMT) and tyramine have also been monitored.
Material and methods
Plant materials and growth conditions and sampling
Germinated seedlings of spring barley elite cultivar Solist (Hordeum vulgare ssp. vulgare, Hv) were transferred to a complete nutrient solution (Ca(NO3)2 × 4H2O 2 Mm; MgSO4 × 7H2O 0.5 Mm; K2SO4 0.7 Mm; KCl 0.1 Mm; KH2PO4 0.1 mM; H3BO3 1 µM, MnSO4 x H2O 0.5 µM; CuSO4 0.2 µM; ZnSO4 × 7H2O 0.5 µM; (NH4)6Mo7O24 × 4H2O 0.01 µM; Fe-EDTA 100 µM) and grown hydroponically under controlled conditions in a climate chamber (14/10 h day/night regime, 24 °C / 19 °C, 70% relative humidity and 250 µmol m−2 s−1 light intensity) as described by Scagliola et al. (2016). After 4 days, roots and the first leaf were sampled, separated, extracted in 1 mL of pure methanol for 24 h under continuous shaking, and filtered with a 0.45 µm filter (Phenomenex, Phenex RC Membrane, 0.45 µm, 4 mm Syringe Filters Non-Sterile, PP Housing, Luer/Slip) as previously described by Maver et al. (2020) until further analysis.
Growth-dependent hordenine biosynthesis
Four days old seedlings were transferred to 50 mL pots containing complete nutrient solutions and monitored for 8 days. In the first 24 h, samples were taken every hour, while for the remaining experimental period, samples were taken three times a day. Both leaves and roots were sampled at each sampling point, separated and extracted in methanol, centrifuged, filtered and analyzed for their hordenine, N-methyltyramine and tyramine content as described earlier.
Nutrient stresses dependent hordenine biosynthesis
Pregerminated seedlings of barley cv Solist were transferred to continuously aerated plant pots (50 mL) containing different nutrient solutions: full nutrient solution (NS), iron deficient (-Fe), nitrogen deficient (-N), sulfur deficient (-S) and phosphorus deficient (-P) nutrient solutions. Barley seedlings were grown for 3 days. Leaves and roots were sampled every day at midday. The same sampling time was chosen to avoid the influence of possible daily biosynthesis variations. After each sampling, roots and leaves were separated, extracted with methanol, centrifuged, filtered and stored at -20 °C until further analysis as described earlier.
Growth-dependent hordenine exudation
Pregerminated seedlings of barley cv Solist were transferred to a full nutrient solution and grown for 3 days. Plants were sampled at the beginning and at the end of these three days following the method described by Maver et al. (2020). Briefly, barley seedlings were transferred to pots containing milliQ-water. The trap solutions were collected after 4 h, filtered with a 0.45 µm filter, freeze-dried, resuspended in pure methanol, and analyzed by HPLC as described earlier.
Hordenine adsorption in soil
Five grams of an agricultural air-dried soil (soil class: Loam; Silt: 39.35%; Clay: 11.08%; Sand: 49.58%; pHH2O: 5.8; C.E.C.: 15.05 [meq/100 g]; Org. Matter DUMAS: 5.95%; field name: Quarryfield and sourced from 56° 27′ 5'' N, 3° 4′ 29'' W. The soil characterization analysis was carried out by James Hutton Institute Mylnefield, Invergowrie, Dundee DD2 5DA) were mixed with 10 mL of 10 mM CaCl2 solution containing hordenine at the following concentrations: 0, 1.5, 5, 10, 15, 20, 30, 60, 100, 150, 200, 250, 300, 400, 600, 800, 1000 mg L−1. Five replicates per concentration were prepared. Soil suspensions were shaken for 24 h at room temperature. Then, suspensions were centrifuged for 5 min at 5000 g, and the supernatant was collected, filtered (0.45 µm, Phenomenex) and analyzed by liquid chromatography (HPLC). Pure reagent grade hordenine was purchased from Sigma-Aldrich (> 99%) and used to prepare two stock solutions in water of 24 mmol L−1 and 1 mmol L−1. Adsorption solutions were prepared by dilution from the stock solution with milliQ water. The adsorption rate of hordenine in soil was obtained by the difference between the initial and final concentration measured in the supernatant by HPLC. Adsorption isotherms of hordenine were fitted applying several nonlinear models: the two-parameter Langmuir and Freundlich isotherms and the three-parameter Sigmoidal Langmuir, Redlich-Peterson and Sips isotherms (Limousin et al. 2007; Foo and Hameed 2010).
Quantitation of hordenine, N-methyltyramine and tyramine
Root extracts were analyzed for their hordenine, N-methyltyramine and tyramine content using an optimized method and instrumental configuration, with a LiChrospher RP-18 column 250 mm × 4.0 mm, 5 µm (Phenomenex, USA) as column and gradient elution with a flow rate of 0.6 mL min−1 as previously described (Maver et al. 2020). The instrumentation consisted of a Waters ALLIANCE HPLC with autosampler coupled with a Waters ACQUITY QDa, equipped with electrospray ionization (ESI) interface in positive ionization (PI) mode. Two mobile phases, A and B, consisted of acetonitrile in 0.1% formic acid (both HPLC grade, Sigma Aldrich) and 0.1 mol L−1 ammonium acetate 0.1% formic acid (Sigma-Aldrich, > 99%) in water, respectively.
The gradient started with 10% A, increased to 50% A within 20 min and returned to 10% A in 5 min, plus other 5 min to stabilize the column. The injected volume was 20 µL. Optimized parameters at the QDa interface were: 120 °C as source temperature, 600 °C as vaporizer temperature, nitrogen at 600 °C as drying gas, cone voltage 15 V, gain 1 and capillary voltage 800 V. SIR modus was adopted by monitoring the m/z 166 for hordenine, m/z 152 for N-methyltyramine, m/z 138 for tyramine and m/z 121 as common main fragments. The retention time for hordenine was 11 min, for N-methyltyramine 7 min and for tyramine 4.5 min. N-methyltyramine standard was purchased from Combi-Blocks (95%), whereas hordenine and tyramine standards from Sigma-Aldrich (> 99%) and stocks solutions were prepared in methanol before using them for the quantifications.
Metabolomics
Sample extracts were filtered using 0.22 μm cellulose syringe filters and collected into amber vials for metabolomics analysis. The screening of metabolites was performed through UHPLC-QTOF mass spectrometry (Agilent Technologies, Santa Clara, CA, USA) with a JetStream electrospray source (UHPLC-ESI/QTOF-MS), according to (Corrado et al. 2020). A 1290 UHPLC chromatographic system that was equipped with a binary pump and a Dual Electrospray Jet Stream ionization system and coupled to a G6550 Q-TOF mass spectrometer was used. Briefly, the chromatographic separation was achieved by using an Agilent Zorbax Eclipse-plus C18 column 100 mm × 2.1 mm, 1.8 μm, and a water and methanol binary gradient. The elution gradient was from 6 to 94% organic within 33 min, the injection volume was 12 µL and the flow rate 200 µL min−1. The mass spectrometer was operated in SCAN mode (100–1200 m/z). The instrument runs in positive polarity, as previously reported (Rouphael et al. 2020; Ceccarelli et al. 2021), to acquiring the highest number of compounds. The nitrogen flow was 14 L/min at a 250 °C gas temperature. The 45 psi nebulizing, nozzle voltage was 350 V nozzle voltage, while the capillary voltage was 4000 V with a scan rate of 1 spectrum/s. Raw spectra were processed in Profinder B.07 (Agilent Technologies, Santa Clara, CA, USA) and annotated using the ‘find-by-formula’ algorithm (i.e., by combining monoisotopic mass, isotope spacing and isotope ratio) as previously reported (Giuberti et al. 2018). Mass (5 ppm) and retention time (0.05 min) alignment were also performed in Profinder B.07. The database PlantCyc 9.6 (Plant Metabolic Network, available in: http://www.plantcyc.org, Schläpfer et al. 2017) was used as a reference for compounds annotation. The annotation strategy corresponded to Level 2 (putatively annotated compounds) of COSMOS Metabolomics Standards Initiative (Salek et al. 2015).
Statistical analysis
Mass Profiler Professional 12.6 (Agilent Technologies) was formerly used for normalization and baselining before chemometrics. Therein, abundances were log2 transformed, normalized at the 75th percentile and then baselined against the median values obtained for each compound of the dataset, according to Salehi et al. (2018). Multivariate statistics were then used for interpretation. The supervised modelling by Orthogonal Projection to Latent Structure Discriminant Analysis (OPLS-DA) was provided by the SIMCA 13 software tool (Umetrics, Malmo, Sweden) in order to discriminate the effect of treatments, according to their metabolic fingerprint. The multivariate model underwent Cross Validation-Analysis of Variance (CV-ANOVA, α = 0.05) and its fitness and prediction ability were evaluated by R2Y and Q2Y parameters, respectively. Finally, One-way ANOVA followed by fold-change analysis was carried out to identify the differential metabolites (α < 0.05, FDR correction; fold-change analysis, FC ≥ 2). Venn representation was performed by with jvenn (http://jvenn.toulouse.inra.fr/app/index.html). Differential metabolites were then imported into the PlantCyc Pathway Tools software (Karp et al. 2009) to computationally detect the secondary biosynthetic pathways involved in the metabolic response against the treatments.
Results
Adsorption isotherms
Soil adsorption isotherms were investigated using, as a model, a soil sampled in Invergowrie, Scotland (UK) used to grow exclusively barley, as previously described in Maver et al. 2021. Among the several nonlinear isotherm models applied to our data (Suppl. Figure 1 and Suppl. Table 1), the three-parameter Sips isotherm, which is a combined form of Langmuir and Freundlich models, resulted in the best fitting with a Residual Sum of Squares (RSSs) of 0.0373; the rest of the isotherm nonlinear models, Freundlich, Langmuir, Redlich-Peterson and Sigmoidal Langmuir, fit with a RSSs of 0.0581, 0.0678, 0,0561 and 0,0663, respectively (Suppl. Figure 1).
Root exudation
All the three compounds investigated, tyramine, N-methyltyramine and hordenine, were detected in the root exudates of barley plants (Table 1). The exudation of all three compounds decreased with time and in particular the concentration of hordenine, and NMT resulted in a tenfold higher concentration compared to tyramine (Table 1). Within three days, a strong decrease of around two orders of magnitude was observed in all three compounds, while the ratio between both hordenine and NMT with tyramine did not vary (approximately 10:1).
Growth dependent short- and long-term accumulation of hordenine, N-methyltyramine and tyramine
It was observed that the hordenine accumulation in roots, together with its precursors, decreased in the first 24 h of 4 DAG (days after germination) seedlings (approx. 40%) (Fig. 2). Yet, the overall content of hordenine and NMT was higher, around one order of magnitude, than tyramine. In addition, for all the compounds, no day-light dependent accumulation was detected within 24 h. The same decreasing trend was observed within eight days of barley root growth (Fig. 3). Here, besides observing the same difference in order of magnitude between hordenine and NMT with tyramine, in terms of content, a very similar pattern of accumulation among the three compounds along all the samplings was observed, revealing an approximate decrease of 90%, 70% and 80% for tyramine, NMT and hordenine respectively, from day 1 to day 8.
Time course of 24 h of hordenine, N-methyltyramine (NMT) and tyramine accumulation in root of barley cv. Solist. Roots were sampled every hour after 4 days of germination. Data are expressed as mean ± SE, n = 5. Letters following the means indicate significant differences, One-way ANOVA with post-hoc Tukey HSD with α = 0.05
Time course of 8 days of hordenine, N-methyltyramine (NMT) and tyramine accumulation in root of barley cv. Solist. Roots were sampled at 8 am, 1 pm and 6 pm for the first 5 days and two samples after 8 days were taken. Data are expressed as mean ± SE, n = 5. Letters following the means indicate significant differences, One-way ANOVA with post-hoc Tukey HSD with α = 0.05
Nutrient stress dependent accumulation of hordenine, N-methyltyramine and tyramine in roots
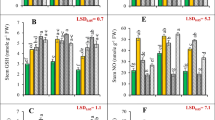
The decreasing trend of hordenine, N-methyltyramine and tyramine in roots was confirmed when barley plants were subjected to nutrient deficiencies, yet with different effects depending on the nutrient deficiency applied, overall ranging from approximately -50% (hordenine and N-methyltyramine) to -70% (tyramine) (Fig. 4 and Suppl. Table 2). Furthermore, hordenine and N-methyltyramine exhibited the same behavior, whereas tyramine followed an independent pattern. After seven days, N deficiency led to the significantly highest concentration of hordenine (+ 230%) and NMT (+ 130%), followed by S (+ 90% and + 45% for hordenine and NMT, respectively) and Fe (+ 80% and + 40% for hordenine and NMT, respectively) deficiency, compared to the control, while P deficient barley plants showed concentrations similar to the control plants. Particularly in N deficient conditions, the content of hordenine and NMT was significantly higher (approx. 50%) in the last sampling after 7 days compared to the sampling at 51 h. Regarding tyramine, only in P deficiency a temporary (at 51 h) but significant increase was observed (approx. 40%), followed by a steep decrease reaching the range detected within the other nutrient deficiencies (Fig. 4 and Suppl. Table 2).
Time course of 3 days of hordenine, N-methyltyramine (NMT) and tyramine accumulation in root of barley cv. Solist under nutrient stresses. Roots at 4 DAG were sampled at 12 am, every day. Nutrient deficiency: control (C), iron (Fe), nitrogen (N), phosphorus (P) and sulfur (S). Data are expressed as mean ± SE, n = 5. Statistical analysis is reported in Suppl. Table 2
Targeted metabolomics
The targeted metabolomic analysis of roots was carried out on 7 days old barley seedlings subjected to different nutrient deficiencies (control, -Fe, -S, -N, -P) in order to get an overview of the abundance of the compounds involved in the hordenine biosynthesis pathway (Fig. 5). Besides hordenine, NMT and tyramine, also candicine (the next product of hordenine) was investigated. This analysis confirmed again the ratio, approximately 10:1, between both hordenine and NMT against tyramine and a significantly higher content of the formers in -N and -P conditions. Also, for candicine, the highest content was observed in -N, yet its overall content among all the conditions was lower than hordenine and NMT.
Metabolomic targeted analysis in roots under nutrient stresses. Metabolomic targeted analysis of tyramine, NMT, hordenine and candicine in barley roots 7 days after germination. Nutrient deficiency: control (C), iron (Fe), nitrogen (N), phosphorus (P) and sulfur (S). Data are expressed as mean area ± SE, n = 3. Letters following the means indicate significant differences, One-way ANOVA with post-hoc Tukey HSD with α = 0.05
Untargeted metabolomics
Besides the compounds involved in hordenine biosynthesis, the whole metabolomic profile of barley roots under the different conditions investigated was also evaluated. Overall, more than 540 metabolites were putatively annotated using the comprehensive database PlantCyc 12.6. The annotated compounds and the corresponding composite mass spectra (mass and abundance combinations) are provided in the Supplementary Dataset v2. Untargeted metabolomics allowed us to discriminate the metabolic profiles of barley roots based on the treatments, thus revealing the distinct effect of nutrient deprivation on the accumulated compounds depending on the element removed. In this regard, the multivariate statistics (i.e., OPLS-DA) showed that the deprivation of N and P had the highest impact on root metabolism, appearing well separated from the control in the score plot, while the effect of Fe and S seemed to be similar to the control (Suppl. Figure 2). Regarding Venn diagrams (Fig. 6a, b), which summarize the compounds differentially modulated by the specific element deficiency, the root response seemed to be element-specific. In fact, only 13 and 3 metabolites were under- and up-accumulated, respectively, as a common response to the nutritional stress (Suppl. Dataset—Table 3).
Metabolomic untargeted analysis in roots under nutrient stresses. Venn diagrams of the differentially accumulating, a) under-accumulated and b) up-accumulated metabolites, in barley root according to the different nutrient deficiencies: iron (Fe), nitrogen (N), phosphorus (P) and sulfur (S). The graphs report the number of common and specific differentially accumulating compounds as c) Overall sum Fold Change biosynthesis, including all the metabolites categories, and as d) sum Fold Change focused on secondary metabolites category. Data are expressed as sum Fold Change per nutrient, n = 3
Afterwards, a pathway analysis was carried out from discriminant compounds (p-value < 0.05; Fold Change (FC) ≥ 2; Suppl. Dataset—Table 4) to unravel the metabolic changes triggered by the nutrient deprivation. In general, P deficiency clearly promoted the accumulation of compounds while S deficiency suppressed the accumulation of metabolites (Fig. 6). For Fe and N deficiency the accumulation trend was less evident.
Considering the nutrient deprivation as an abiotic stress and taken into account that secondary metabolism is stimulated under adverse conditions, secondary metabolites were further studied. Figure 6d represents this metabolomic analysis and depicts the principal classes of secondary metabolites involved in response to nutrient deficiencies. In detail, the deprivation of P and, to a lesser extend N deprivation, caused an up-accumulation of secondary metabolites in barley roots, i.e. phenylpropanoids (Suppl. Table 5). In particular the biosynthesis of alkaloids and flavonoids was elicited by N and P deficiency and decreased in the absence of S. Similar trend was observed for phytoalexins that were in general promoted by N and P deficiency while were repressed by Fe and S shortage. Although terpenes seemed to be less implicated in the complex response of roots to stress, N and P deprivation similarly promoted their accumulation (Fig. 6d).
Although the S deficiency seemed to have a moderate effect on the root metabolome, as observed in the OPLS-DA (Suppl. Figure 2), the impact on secondary metabolism was strongly marked. In fact, S deficiency provoked a decrease of most of secondary metabolites compared to the control. Although the effect of Fe deficiency was less pronounced, this shortage negatively affected phenylpropanoids accumulation, while promoting the biosynthesis of several N-containing secondary metabolites.
Discussion
The allelochemical hordenine, along with gramine, is one of the main alkaloids found in barley and has already been demonstrated for its allelopathic traits (Kremer and Ben-Hammouda 2009). Although the hordenine biosynthetic pathway has been already described (Schenck and Maeda 2018) (Fig. 1), little is still known regarding its root exudation pattern, including that of its precursors, N-methyltyramine and tyramine. Similarly, there is no evidence whether the accumulation patterns of these compounds change with plant development (in the short and long term) or are affected by the nutritional state of the plant, with reference to N, P, S or Fe.
Hordenine and gramine are released by barley as root exudates like previously reported (Lovett et al 1994; Maver et al 2020), even though the trans-membrane mechanisms underlying the process have not been well characterized and fully understood yet (Weston et al. 2012; Lebecque et al. 2018). Data here reported confirm this phenomenon but also revealed the presence of the precursors N-methyltyramine (NMT) and tyramine in addition to hordenine in the root exudates of barley cv. Solist (Table 1) (Schenck and Maeda 2018). However, the exudation of all the three compounds decreased significantly within three days of barley growth (Table 1), underlining their possible specific role in the first phase of plant development. Indeed, it is very likely that these allelochemicals help the seedlings to be more competitive towards nearby plants by allelopathy or in establishing interactions with neighboring plants and/or microorganisms in the rhizosphere (Bertin et al. 2003; Latif et al. 2017; Maver et al. 2021). Moreover, the overall exudation pattern of tyramine was approximately 10 times lower than NMT and hordenine (Table 1). This difference in terms of concentration could be ascribed to a greater involvement of tyramine into other metabolic pathways, including for example the production of defense hydroxycinnamic acid amides for cell wall reinforcement or acting as a precursor for other secondary metabolites/alkaloids (e.g., benzylisoquinoline). Depending on the plant development stage or defense response, these other secondary metabolites may be more necessary, having higher priority and/or be also less energy demanding in their biosynthesis with respect to hordenine in barley roots (Ishihara et al. 2017; Schenck and Maeda 2018).
Allelochemicals can be released into the rhizosphere, and their concentration, mobility, and activity are prerequisites to fulfill their allelopathic functions against target plants. Indeed, root exudates, once in the rhizosphere, might undergo several processes which modify them considerably, e.g., microbial degradation, oxidation, or reversible/irreversible binding to soil minerals, altering their persistence and bioavailability (Mimmo et al. 2014; Latif et al. 2017). With respect to allelochemicals, it has been observed that benzoxazinoids (Macías et al. 2004) or phenolics acids (Tharayil et al. 2008) are more persistent in soils reaching half-lives of more than several days. Since hordenine and its precursors share similar chemical properties and a low water solubility (like benzoxazinoids and phenolic acids), it is very likely that they also share a similar low turnover. However, it is well known that the availability level of an exudate in the rhizosphere depends also on soil sorption reactions. Hence, the hordenine behavior in soil (availability and mobility) has been assessed by investigating i) the adsorption and ii) distribution of the molecule in agricultural soil, and iii) estimating the soil adsorption coefficient (Kfoc) related to the soil organic matter content (Suppl. Figure 1 and Suppl. Table 1). In this study, the best fitting isotherm model was the three-parameters Sips, which is a combination of the Freundlich and Langmuir models (Foo and Hameed 2010), and resulting in a sigmoidal curve. This curve can describe a cooperative adsorption phenomenon, very common for non-polar organic compounds like hordenine or gramine (Maver et al. 2021), presenting at least two opposite mechanisms: low affinity for the soil at low concentrations of the alkaloid and high levels of affinity with increasing concentrations (Sparks 2003; Limousin et al. 2007). Moreover, to measure the mobility of hordenine in soil, Kfoc, defined as organic-carbon normalized Freundlich distribution coefficient, was estimated following the OECD guideline 121 (OECD 2001), resulting as 384, thus classifying hordenine as moderately mobile in soil according to FAO Mobility Classification (FAO 2000).
Allelochemicals, as part of secondary metabolites, can be mainly produced in early plant development phases and also preferentially accumulated in specific plant tissues or organs, conferring advantageous traits to the seedlings in the most vulnerable initial phases of any plant species, e.g., in terms of survivability, competitiveness, and/or interaction with the environment (De-La-Cruz Chacón et al. 2013). In our study, we observed a very similar behavior for hordenine and its two precursors in barley roots. In fact, their content considerably decreased within the first days (Figs. 2 and 3). A similar phenomenon has been already described for hordenine in barley (Lovett et al. 1994; Maver et al. 2020), yet no information is available concerning the first days of plant growth. In addition, all the three compounds involved in the hordenine biosynthetic pathway decreased by approx. -40% in 24 h (Fig. 2) and by up to approx. -80% after eight days (with approx. -20% decrease per day) (Fig. 3). These results highlight a strong decrease within the very early phases of the plant growth and also confirms a very similar trend of reduction among the compounds investigated and in line with other allelochemicals reported in several other plants (De-La-Cruz Chacón et al. 2013). However, tyramine behaved slightly differently than hordenine and NMT in both the 24 h time course (TC) (Fig. 2) and the eight days TC (Fig. 3), as was also observed in the root exudation profile (Table 1) and targeted metabolomics (Fig. 5). Again, this trend is most likely due to the involvement of tyramine in other metabolic pathways, as described earlier.
With respect to the external modulation factors of the biosynthetic pathway, it should be noted that abiotic stresses, like the nutrient deficiencies, could very likely act as trigger and/or affect the synthesis, accumulation and, then, release into the environment of these alkaloids (Gershenzon 1984; Albuquerque et al. 2011). To the best of our knowledge, there is no literature available regarding the relationship between nutrient stress and allelochemicals accumulation in barley roots. The shortage of some essential nutrients (e.g., N, P, S and Fe) is perceived by the plants as an abiotic stress limiting considerably the growth rate. As a consequence, these plants, suffering from the nutritional disorder, might induce the production of allelochemicals as i) defense mechanisms (e.g., triggered production of hordenine and gramine under pathogens infections) (Matsuo et al. 2001; Ishihara et al. 2017), ii) temporary nutrient storages (e.g., alkaloids and cyanogenic glycosides) or iii) facilitating nutrients mobilization and uptake (e.g., phenolics) (Herms and Mattson 1992; Cao et al. 2020). In this study, a nutrient stress dependent accumulation of hordenine, N-methyltyramine and tyramine in barley roots was evaluated by a time-dependent approach, targeted metabolomic, and eventually untargeted metabolomic analysis. In general, the pattern of all the three metabolites followed the same decreasing trend in 24 h and 8 days (Figs. 2 and 3), although the downward trend is slightly different depending on the nutrient deficiency applied (Fig. 4). Moreover, tyramine, as observed in full nutrient solutions (Figs. 2 and 3), exhibited a more pronounced overall decreasing trend (approx. -75%) with respect to hordenine and NMT (approx. -50%) (Fig. 4). Furthermore, the accumulation of hordenine and NMT was significantly enhanced under N deficiency both in the TC analysis and the targeted metabolomics (Figs. 4 and 5). Candicine, one of the possible downstream products of the hordenine pathway and characterized by an extra methylation step (Servillo et al. 2012), was enhanced in N deficient conditions (Fig. 5).
This aspect was also investigated analyzing the biosynthesis fold change of several categories of metabolites in roots, applying an untargeted metabolomic approach, within all the nutrient deficiencies tested (Fig. 6). Since hordenine, NMT and tyramine are all N containing secondary metabolites, it is worthwhile noticing how this category of compounds was the most intensively modulated, followed by fatty acids and lipids. In particular, S deficiency induced an overall distinctive under-accumulation, while P shortage induced an up-accumulation of secondary metabolites (Fig. 6 and Suppl. Table 5). Sulfur and P are both essential macronutrients for barley and their absence or reduced availability trigger specific responses within primary and secondary metabolisms (Amtmann and Armengaud 2009). For instance, under S deficiency, a general decrease of lipids, RNA, amino acids (Cys and Met), chlorophyll, S-containing compounds like S-adenosyl-methionine (SAM) and secondary metabolites like glucosinolates was already reported in A. thaliana (Nikiforova et al. 2005; Amtmann and Armengaud 2009). Our data confirm these results for barley, highlighting how the involvement of SAM in many SAM-dependent methyltransferases as methyl donor, is crucial for the biosynthesis of secondary metabolites or polyamines (Lewandowska and Sirko 2008). On the contrary, to cope with P deficiency, plants generally start recycling P from organic molecules, for instance reducing protein biosynthesis but increasing protein degradation, yet leading to an ammonium toxicity due to a higher concentration of free amino acids (Huang et al. 2008). Barley, an ammonium sensitive species (Britto and Kronzucker 2002), may mobilize it into N related metabolites, such as tyramine in roots, to reduce its level (Huang et al. 2008; Pant et al. 2015). This could eventually explain the slight increment of tyramine content during the time course (Fig. 4). Moreover, there are phenylpropanoids, flavonols and glucosinolates, among the secondary metabolites reported to be up-accumulated under P deficiency (Pant et al. 2015).
Regarding N and Fe instead, there are less clear trends of accumulation (Fig. 6). Interestingly, in N deficient conditions both hordenine and NMT relative abundances are slightly increased in the last sampling day of the time course analysis (Fig. 4). These results confirm the general positive trend of the secondary metabolites, which are already known for being involved in plants responses to N starvation (Cao et al. 2020).
Conclusions
While the regulation mechanisms of the alkaloid hordenine pathway remain unelucidated, all the performed experiments in this study have widely contributed to a better understanding of the production, accumulation, and release patterns of hordenine, N-methyltyramine and tyramine in barley root sampling. Furthermore, the results revealed that nutrient deficiencies induced different production and accumulation patterns of all the three metabolites in modern barley roots. In particular, our results also suggest that a specific nutrient availability might trigger and/or affect the biosynthesis response of the secondary metabolites and in particular of the three compounds investigated.
Data availability
The datasets generated during and/or analyzed during the current study, along with the code used to generate all the figures reported, are publicly available in the GitHub repository, available at https://github.com/Stramon1um/hordenine_solist.
References
Albuquerque MB, Santos RC, Lima LM et al (2011) Allelopathy, an alternative tool to improve cropping systems. A Review Agron Sustain Dev 31:379–395. https://doi.org/10.1051/agro/2010031
Amtmann A, Armengaud P (2009) Effects of N, P, K and S on metabolism: new knowledge gained from multi-level analysis. Curr Opin Plant Biol 12:275–283. https://doi.org/10.1016/j.pbi.2009.04.014
Belz RG (2007) Allelopathy in crop/weed interactions — an update. Pest Manag Sci 63:308–326. https://doi.org/10.1002/ps.1320
Bertholdsson NO (2004) Variation in allelopathic activity over 100 years of barley selection and breeding. Weed Res 44:78–86. https://doi.org/10.1111/j.1365-3180.2003.00375.x
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159:567–584. https://doi.org/10.1078/0176-1617-0774
Cao Y-w, Qu R-j, Tang X-q et al (2020) UPLC-Triple TOF-MS/MS based metabolomics approach to reveal the influence of nitrogen levels on Isatis indigotica seedling leaf. Sci Hortic (Amsterdam) 266. https://doi.org/10.1016/j.scienta.2020.109280
Ceccarelli AV, Miras-Moreno B, Buffagni V et al (2021) Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 10:326. https://doi.org/10.3390/plants10020326
Corrado G, Lucini L, Miras-Moreno B et al (2020) Metabolic insights into the anion-anion antagonism in sweet basil: Effects of different nitrate/chloride ratios in the nutrient solution. Int J Mol Sci 21:2482. https://doi.org/10.3390/ijms21072482
De-La-Cruz Chacón I, Riley-Saldaña CA, González-Esquinca AR (2013) Secondary metabolites during early development in plants. Phytochem Rev 12:47–64
FAO (2000) FAO PESTICIDE DISPOSAL SERIES 8 Assessing soil contamination A reference manual
Farooq M, Jabran K, Cheema ZA et al (2011) The role of allelopathy in agricultural pest management. Pest Manag Sci 67:493–506. https://doi.org/10.1002/ps.2091
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/J.CEJ.2009.09.013
Gershenzon J (1984) Changes in the levels of plant secondary metabolites under water and nutrient stress. Phytochem Adapt Stress: 273–320. https://doi.org/10.1007/978-1-4684-1206-2_10
Giuberti G, Rocchetti G, Sigolo S et al (2018) Exploitation of alfalfa seed (Medicago sativa L.) flour into gluten-free rice cookies: Nutritional, antioxidant and quality characteristics. Food Chem 239:679–687. https://doi.org/10.1016/j.foodchem.2017.07.004
Hanson AD, Ditz KM, Singletary GW, Leland TJ (1983) Gramine accumulation in leaves of barley grown under high-temperature stress. Plant Physiol 71:896–904. https://doi.org/10.1104/pp.71.4.896
Herms DA, Mattson WJ (1992) The dilemma of plants: To grow or defend. Q Rev Biol 67:283–335. https://doi.org/10.1086/417659
Huang CY, Roessner U, Eickmeier I et al (2008) Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol 49:691–703. https://doi.org/10.1093/pcp/pcn044
IAS IAS (1996) First World Congress on Allelopathy, a Science for the Future
Ishiai S, Kondo H, Hattori T et al (2016) Hordenine is responsible for plant defense response through jasmonate-dependent defense pathway. Physiol Mol Plant Pathol 96:94–100. https://doi.org/10.1016/j.pmpp.2016.10.003
Ishihara A, Kumeda R, Hayashi N et al (2017) Induced accumulation of tyramine, serotonin, and related amines in response to Bipolaris sorokiniana infection in barley. Biosci Biotechnol Biochem 81:1090–1098. https://doi.org/10.1080/09168451.2017.1290520
Jabran K (2017) Manipulation of Allelopathic Crops for Weed Control
Karp PD, Paley SM, Krummenacker M et al (2009) Pathway Tools version 13.0: Integrated software for pathway/genome informatics and systems biology. Brief Bioinform 11:40–79. https://doi.org/10.1093/bib/bbp043
Kim S-C, Lee J-H, Kim M-H et al (2013) Hordenine, a single compound produced during barley germination, inhibits melanogenesis in human melanocytes. Food Chem 141:174–181. https://doi.org/10.1016/J.FOODCHEM.2013.03.017
Kong CH, Chen XH, Hu F, Zhang SZ (2011) Breeding of commercially acceptable allelopathic rice cultivars in China. Pest Manag Sci 67:1100–1106. https://doi.org/10.1002/ps.2154
Kremer RJ, Ben-Hammouda M (2009) Allelopathic plants. 19. Barley (hordeum vulgare L). Allelopath J 24:225–242
Larsson KAE, Zetterlund I, Delp G, Jonsson LMV (2006) N-Methyltransferase involved in gramine biosynthesis in barley: Cloning and characterization. Phytochemistry 67:2002–2008. https://doi.org/10.1016/j.phytochem.2006.06.036
Latif S, Chiapusio G, Weston LA (2017) Allelopathy and the Role of Allelochemicals in Plant Defence. Elsevier Ltd
Lebecque S, Crowet JM, Lins L et al (2018) Interaction between the barley allelochemical compounds gramine and hordenine and artificial lipid bilayers mimicking the plant plasma membrane. Sci Rep 8:1–13. https://doi.org/10.1038/s41598-018-28040-6
Lewandowska M, Sirko A (2008) Recent advances in understanding plant response to sulfur-deficiency stress. Acta Biochim Pol 55:457–471. https://doi.org/10.18388/abp.2008_3051
Limousin G, Gaudet JP, Charlet L et al (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochemistry 22:249–275. https://doi.org/10.1016/j.apgeochem.2006.09.010
Lovett JV, Hoult AHCC, Christen O (1994) Biologically active secondary metabolites of barley. IV. Hordenine production by different barley lines. J Chem Ecol 20:1945–1954. https://doi.org/10.1007/BF02066235
Lovett JV, Hoult AHC (1995) Allelopathy and Self-Defense in Barley. In: Allelopathy. ACS Publications, pp 170–183
Macías FA, Oliveros-Bastidas A, Marín D et al (2004) Degradation studies on benzoxazinoids. Soil degradation dynamics of 2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) and its degradation products, phytotoxic allelochemicals from gramineae. J Agric Food Chem 52:6402–6413. https://doi.org/10.1021/jf0488514
Matsuo H, Taniguchi K, Hiramoto T et al (2001) Gramine increase associated with rapid and transient systemic resistance in barley seedlings induced by mechanical and biological stresses. Plant Cell Physiol 42:1103–1111
Maver M, Escudero-Martinez C, Abbott J et al (2021) Applications of the indole-alkaloid gramine modulate the assembly of individual members of the barley rhizosphere microbiota. PeerJ 9:e12498. https://doi.org/10.7717/peerj.12498
Maver M, Miras-Moreno B, Lucini L et al (2020) New insights in the allelopathic traits of different barley genotypes: middle Eastern and Tibetan wild-relative accessions vs. cultivated modern barley. PLoS One 15:1–18. https://doi.org/10.1371/journal.pone.0231976
Mimmo T, Del Buono D, Terzano R et al (2014) Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur J Soil Sci 65:629–642. https://doi.org/10.1111/ejss.12158
Nikiforova VJ, Kopka J, Tolstikov V et al (2005) Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of arabidopsis plants 1[w]. Plant Physiol 138:304–318. https://doi.org/10.1104/pp.104.053793
OECD (2001) OECD Guidline for testing chemicals Estimation of the Adsorption Coefficient ( K oc ) on Soil and on Sewage Sludge using High Performance Liquid Chromatography (HPLC) 121
Oveisi M, Mashhadi HR, Baghestani MA et al (2008) Assessment of the allelopathic potential of 17 Iranian barley cultivars in different development stages and their variations over 60 years of selection. Weed Biol Manag 8:225–232. https://doi.org/10.1111/j.1445-6664.2008.00301.x
Overland L (1966) The role of allelopathic substances in the “smother crop” barley. Am J Bot 53:423. https://doi.org/10.2307/2440341
Pant BD, Pant P, Erban A et al (2015) Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ 38:172–187. https://doi.org/10.1111/pce.12378
Rouphael Y, Lucini L, Miras-Moreno B et al (2020) Metabolomic responses of maize shoots and roots elicited by combinatorial seed treatments with microbial and non-microbial biostimulants. Front Microbiol 11:664. https://doi.org/10.3389/fmicb.2020.00664
Salehi H, Chehregani A, Lucini L et al (2018) Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci Total Environ 616–617:1540–1551. https://doi.org/10.1016/j.scitotenv.2017.10.159
Salek RM, Neumann S, Schober D et al (2015) COordination of Standards in MetabOlomicS (COSMOS): facilitating integrated metabolomics data access. Metabolomics 11:1587–1597. https://doi.org/10.1007/s11306-015-0810-y
Scagliola M, Pii Y, Mimmo T et al (2016) Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant Physiol Biochem 107:187–196. https://doi.org/10.1016/j.plaphy.2016.06.002
Schenck CA, Maeda HA (2018) Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 149:82–102. https://doi.org/10.1016/j.phytochem.2018.02.003
Schläpfer P, Zhang P, Wang C et al (2017) Genome-wide prediction of metabolic enzymes, pathways, and gene clusters in plants. Plant Physiol 173:2041–2059. https://doi.org/10.1104/pp.16.01942
Servillo L, Giovane A, Balestrieri ML et al (2012) N ‑ Methylated Tryptamine Derivatives in Citrus Genus Plants
Sparks DL (2003) Sorption phenomena on soils. Environ Soil Chem: 133–186. https://doi.org/10.1016/b978-012656446-4/50005-0
Tharayil N, Bhowmik PC, Xing B (2008) Bioavailability of allelochemicals as affected by companion compounds in soil matrices. J Agric Food Chem 56:3706–3713. https://doi.org/10.1021/jf073310a
Vasilakoglou I, Dhima K, Lithourgidis A, Eleftherohorinos I (2009) Allelopathic potential of 50 barley cultivars and the herbicidal effects of barley extract. Allelopath J 24:309–320
Weston LA, Ryan PR, Watt M (2012) Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J Exp Bot 63:3445–3454. https://doi.org/10.1093/jxb/ers054
Whittaker RH, Feeny PP (1971) Allelochemics: Chemical Interactions between Species. Science (80-. ) 171:757–770
Funding
Open access funding provided by Libera Università di Bolzano within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Mauro Maver, Fabio Trevisan, Tanja Mimmo contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mauro Maver, Fabio Trevisan and Begoña Miras-Moreno. The first draft of the manuscript was written by Mauro Maver and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: Martin Weih.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11104_2022_5553_MOESM2_ESM.xlsx
Supplementary file2 Supplementary Dataset. List of the overall metabolites annotated using the comprehensive database PlantCyc 12.6 in barley roots under nutrient stress. The annotated compounds and the corresponding composite mass spectra (mass and abundance combinations) are here provided among the nutrient deficiencies tested: control (C), iron (Fe), nitrogen (N), phosphorus (P) and sulfur (S) (XLSX 173 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maver, M., Trevisan, F., Miras-Moreno, B. et al. The interplay between nitrogenated allelochemicals, mineral nutrition and metabolic profile in barley roots. Plant Soil 479, 715–730 (2022). https://doi.org/10.1007/s11104-022-05553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05553-8