Abstract
Aims
The effect of Eucalyptus stump substrate decomposition on microbial resource limitation driving factors are unclear.
Methods
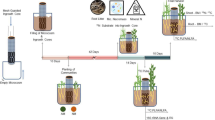
Eucalyptus stump substrate samples from five decay classes with contrasting qualities were decomposed in Eucalyptus undergrowth soil for three months in a laboratory microcosm experiment. We determined the substrate quality, microbial biomass, microbial community structure, and extracellular enzyme activities for the decay classes of stump substrates.
Results
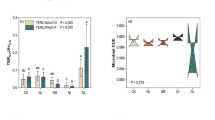
The stump carbon:nitrogen (C:N), carbon:phosphorus (C:P), and nitrogen:phosphorus (N:P) ratios and dissolved organic carbon:total dissolved nitrogen (DOC:TDN), dissolved organic carbon:available phosphorus (DOC:AP), and total dissolved nitrogen:available phosphorus (TDN:AP) ratios increased and then decreased as the decay class increased, whereas the C:N and C:P imbalances between the microorganisms inhabiting stumps and their resources increased. The activities of N- and P-acquiring enzymes improved as the decay class increased, and such changes improved the microbial N and P limitation of stumps and reduced the microbial C use efficiency. In advanced decay classes, the microbial community structure shifted toward a higher ratio of fungi to bacteria and a lower ratio of gram-positive bacteria to gram-negative bacteria. Redundancy analysis showed that P, DOC, and DOC:AP were all closely related to the enzyme activities, whereas P and N were the critical factors alter microbial composition community. In addition, ecoenzymatic stoichiometry and the C:N:P imbalance played key roles on regulating microbial C use efficiency.
Conclusions
The decomposition of Eucalyptus stump substrates modified microbial communities and enzymatic stoichiometry, leading to microbial growth from P limitation to N limitation.






Similar content being viewed by others
References
Adamczyk B, Kilpeläinen P, Kitunen V, Smolander A (2014) Potential activities of enzymes involved in N, C, P and S cycling in boreal forest soil under different tree species. Pedobiologia 57(2):97–102
Andresen LC, Dungait JAJ, Bol R, Selsted MB, Ambus P, Michelsen A, Smidt H (2014) Bacteria and fungi respond differently to multifactorial climate change in a temperate heathland, traced with 13C-glycine and FACE CO2. PLoS ONE 9(1):e85070
Ashagrie Y, Zech W, Guggenberger G (2005) Transformation of a Podocarpus falcatus dominated natural forest into a monoculture Eucalyptus globulus plantation at Munesa, Ethiopia: Soil organic C, N and S dynamics in primary particle and aggregate-size fractions. Agr Ecosyst Environ 106(1):89–98
Bače R, Svoboda M, Pouska V, Janda P, Cervenka J (2012) Natural regeneration in Central-European subalpine spruce forests: Which logs are suitable for seedling recruitment? Forest Ecol Manag 266:254–262
Bai X, Dippold MA, An S, Wang B, Zhang H, Loeppmann S (2021) Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol Indic 121:107200
Bastida F, Moreno JL, Hernández T, García C (2007) Microbial activity in non-agricultural degraded soils exposed to semiarid climate. Sci Total Environ 378(1–2):183–186
Bastida F, Kandeler E, Hernandez T, Garcia C (2008) Long-term effect of municipal solid waste amendment on microbial abundance and humus-associated enzyme activities under semiarid conditions. Microb Ecol 55(4):651–661
Billings SA, Ziegler SE (2008) Altered patterns of soil carbon substrate usage and heterotrophic respiration in a pine forest with elevated CO2 and N fertilization. Global Change Biol 14(5):1025–1036
Błońska E, Kacprzyk M, Spólnik A (2017) Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol Res 32(2):193–203
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants-An economic analogy. Annu Rev Ecol Evol S 16:363–392
Bradford MA, Warren RJ, Baldrian P, Crowther TW, Maynard DS, Oldfield EE, Wieder WR, Wood SA, King JR (2014) Climate fails to predict wood decomposition at regional scales. Nat Clim Change 4(7):625–630
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14(4):319–329
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Chavez-Vergara B, Merino A, Vázquez-Marrufo G, García-Oliva F (2014) Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico. Geoderma 235:133–145
Chen H, Li DJ, Xiao K, Wang K, Treseder K (2018) Soil microbial processes and resource limitation in karst and non-karst forests. Funct Ecol 32(5):1400–1409
Chen Y, Ma S, Jiang H, Hu Y, Lu X (2020) Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 377:114577
Chen Q, Liu Z, Zhou J, Xu X, Zhu Y (2021) Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize-wheat rotation on China’s Loess Plateau. Sci Total Tnviron 775:145930
Cleveland CC, Liptzin D (2007) C:N: P stoichiometry in soil: Is there a “redfield ratio” for the microbial biomass? Biogeochemistry 85(3):235–252
Cocciufa C, Gerth W, Luiselli L, Redolfi De Zan L, Cerretti P, Carpaneto GM (2014) Survey of saproxylic beetle assemblages at different forest plots in central Italy. B Insectol 67(2):295–306
Dong H, Ge J, Sun K, Wang B, Xue J, Wakelin SA, Wu J, Sheng W, Liang C, Xu Q, Jiang P, Chen J, Qin H (2021) Change in root-associated fungal communities affects soil enzymatic activities during Pinus massoniana forest development in subtropical China. Forest Ecol Manag 482:118817
Drenovsky RE, Vo D, Graham KJ, Scow KM (2004) Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb Ecol 48(3):424–430
Du E, Terrer C, Pellegrini AF, Ahlstrom A, van Lissa CJ, Zhao X, Xia N, Wu X, Jackson RB, Lab LLN, Llnl LCUS (2020) Global patterns of terrestrial nitrogen and phosphorus limitation. Nat Geosci 13(3):221–226
Ekblad A, Nordgren A (2002) Is growth of soil microorganisms in boreal forests limited by carbon or nitrogen availability? Plant Soil 242(1):115–122
Fanin N, Fromin N, Buatois B, Hättenschwiler S (2013) An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol Lett 16(6):764–772
Fanin N, Moorhead D, Bertrand I, Sveriges L (2016) Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 129(1–2):21–36
Fernández-Alonso MJ, Yuste JC, Kitzler B, Ortiz C, Rubio A (2018) Changes in litter chemistry associated with global change-driven forest succession resulted in time-decoupled responses of soil carbon and nitrogen cycles. Soil Biol Biochem 120:200–211
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88(6):1354–1364
Freschet GT, Weedon JT, Aerts R, van Hal JR, Cornelissen JH (2012) Interspecific differences in wood decay rates: Insights from a new short-term method to study long-term wood decomposition. J Ecol 100(1):161–170
Frost PC, Benstead JP, Cross WF, Hillebrand H, Larson JH, Xenopoulos MA, Yoshida T (2006) Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol Lett 9(7):774–779
Frostegard A, Tunlid A, Baath E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43(8):1621–1625
Gessner MO, Swan CM, Dang CK, Mckie BG, Bardgett RD, Wall DH, Hättenschwiler S, Sveriges L (2010) Diversity meets decomposition. Trends Ecol Evol 25(6):372–380
Giacometti C, Cavani L, Baldoni G, Ciavatta C, Marzadori C, Kandeler E (2014) Microplate-scale fluorometric soil enzyme assays as tools to assess soil quality in a long-term agricultural field experiment. Appl Soil Ecol 75:80–85
Grayston SJ, Prescott CE (2005) Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Biol Biochem 37(6):1157–1167
Guo K, Zhao Y, Liu Y, Chen J, Wu Q, Ruan Y, Li S, Shi J, Zhao L, Sun X, Liang C, Xu Q, Qin H (2020) Pyrolysis temperature of biochar affects ecoenzymatic stoichiometry and microbial nutrient-use efficiency in a bamboo forest soil. Geoderma 363:114162
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Cummins KW (1986) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15(C):133–302
Hu Z, Michaletz ST, Johnson DJ, Mcdowell NG, Huang Z, Zhou X, Xu C (2018) Traits drive global wood decomposition rates more than climate. Global Change Biol 24(11):5259–5269
Hunter ML, Schmiegelow FK (1990) Wildlife, forests and forestry: Principles of managing forests for biological diversity 2(nd) edition. Forest Chron 87(4):570
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36(1):5–7
Kaiser C, Franklin O, Dieckmann U, Richter A (2014) Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol Lett 17(6):680–690
Koranda M, Kaiser C, Fuchslueger L, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2014) Fungal and bacterial utilization of organic substrates depends on substrate complexity and N availability. Fems Microbiol Ecol 87(1):142–152
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol Biochem 40(2):425–433
Laiho R, Prescott CE (2004) Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: A synthesis. Can J Forest Res 34(4):763–777
Lassauce A, Paillet Y, Jactel H, Bouget C (2011) Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol Indic 11(5):1027–1039
Li J, Dong L, Liu Y, Wu J, Wang J, Shangguan Z, Deng L (2022a) Soil organic carbon variation determined by biogeographic patterns of microbial carbon and nutrient limitations across a 3, 000-km humidity gradient in China. CATENA 209:105849
Li J, Xie J, Zhang Y, Dong L, Shangguan Z, Deng L (2022b) Interactive effects of nitrogen and water addition on soil microbial resource limitation in a temperate desert shrubland. Plant Soil 1–18
Maas GC, Sanquetta CR, Marques R, Machado SD, Sanquetta MN, Corte AP, Schmidt LN (2021) Combining sample designs to account for the whole necromass carbon stock in brazilian atlantic forest. J Sustain Forest 40:639–655
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science (New York N Y) 321(5889):684–686
Manzoni S, Taylor P, Richter A, Porporato A, Agren GI, Sveriges L (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196(1):79–91
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front Microbiol 4:223
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014a) Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014b) Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Naesset E (1999) Relationship between relative wood density of Picea abies logs and simple classification systems of decayed coarse woody debris. Scand J For Res 14(5):454–461
Paletto A, Tosi V (2010) Deadwood density variation with decay class in seven tree species of the Italian Alps. Scand J For Res 25(2):164–173
Paterson E, Osler G, Dawson LA, Gebbing T, Sim A, Ord B (2008) Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol Biochem 40(5):1103–1113
Persiani AM, Audisio P, Lunghini D, Maggi O, Granito VM, Biscaccianti AB, Chiavetta U, Marchetti M (2010) Linking taxonomical and functional biodiversity of saproxylic fungi and beetles in broad-leaved forests in southern Italy with varying management histories. Plant Biosyst 144(1):250–261
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil. A theoretical model. Soil Biol Biochem 35(4):549–563
Schindler DW (2003) Ecological stoichiometry: The biology of elements from molecules to the biosphere. Nature 423(6937):225–226
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64(5):1659–1668
Shimizu K (2013) Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. Isrn Biochemistry 2013:645983
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795-U117
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol Lett 16(7):930–939
Sinsabaugh RL, Turner BL, Talbot JM, Waring BG, Powers JS, Kuske CR, Moorhead DL, Follstad Shah JJ (2016) Stoichiometry of microbial carbon use efficiency in soils. Ecol Monogr 86(2):172–189
Spohn M, Klaus K, Wanek W, Richter A (2016) Microbial carbon use efficiency and biomass turnover times depending on soil depth-Implications for carbon cycling. Soil Biol Biochem 96:74–81
Stark S, Männistö MK, Eskelinen A (2014) Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 383(1–2):373–385
Tapia-Torres Y, Elser JJ, Souza V, García-Oliva F (2015) Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol Biochem 87:34–42
Tláskal V, Brabcová V, Větrovský T, Jomura M, López-Mondéjar R, Monteiro LM, Saraiva JP, Human ZR, Cajthaml T, Da Rocha UN, Baldrian P (2021) Complementary roles of Wood-Inhabiting fungi and bacteria facilitate deadwood decomposition. mSystems 6(1):e01078-20
Ullah S, Ai C, Huang S, Zhang J, Jia L, Ma J, Zhou W, He P (2019) The responses of extracellular enzyme activities and microbial community composition under nitrogen addition in an upland soil. PLoS ONE 14(9):e223026
Uselman SM, Qualls RG, Lilienfein J (2012) Quality of soluble organic C, N, and P produced by different types and species of litter: Root litter versus leaf litter. Soil Biol Biochem 54:57–67
van Geffen KG, Poorter L, Sass-Klaassen U, Van Logtestijn RS, Cornelissen JH (2010) The trait contribution to wood decomposition rates of 15 Neotropical tree species. Ecology 91(12):3686–3697
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707
Waring BG (2013) Exploring relationships between enzyme activities and leaf litter decomposition in a wet tropical forest. Soil Biol Biochem 64:89–95
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117(1):101–113
Weedon JT, Cornwell WK, Cornelissen JH, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol Lett 12(1):45–56
Wei X, Zhu Z, Liu Y, Luo Y, Deng Y, Xu X, Liu S, Richter A, Shibistova O, Guggenberger G, Wu J, Ge T (2020) C:N: P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol Fert Soils 56(8):1093–1107
Wild B, Schnecker J, Knoltsch A, Takriti M, Mooshammer M, Gentsch N, Mikutta R, Alves RJ, Gittel A, Lashchinskiy N, Richter A (2015) Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Global Biogeochem Cy 29(5):567–582
Xu Q, Liang C, Chen J, Li Y, Qin H, Fuhrmann J (2020) Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes. Glob Ecol Conserv 21:e00787
You Y, Xu H, Wu X, Zhou X, Tan X, Li M, Wen Y, Zhu H, Cai D, Huang X (2020) Native broadleaf tree species stimulate topsoil nutrient transformation by changing microbial community composition and physiological function, but not biomass in subtropical plantations with low P status. Forest Ecol Manag 477:118491
Yuan X, Niu D, Gherardi LA, Liu Y, Wang Y, Elser JJ, Fu H (2019) Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol Biochem 138:107580
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol Monogr 85(2):133–155
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol Fert Soils 29(2):111–129
Zhu Z, Ge T, Luo Y, Liu S, Xu X, Tong C, Shibistova O, Guggenberger G, Wu J (2018) Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol Biochem 121:67–76
Funding
This research was provided by the “Dynamic changes in microbial communities during decomposition of Eucalyptus stumps based on ecological stoichiometry” (2017GXNSFAA198007) from the Guangxi Natural Science Foundation, “Characteristics of microbial communities during decomposition of underground coarse roots of Eucalyptus stumps” (32160359) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Alfonso Escudero.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, X., Cheng, F., Li, M. et al. Effect of different decay classes of Eucalyptus stump substrates on microbial resource limitation and carbon-use efficiency. Plant Soil 478, 651–669 (2022). https://doi.org/10.1007/s11104-022-05499-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05499-x