Abstract
Background
About 30 % of vegetables in China are produced in intensively managed greenhouses comprising flood irrigation and extreme rates of nitrogen fertilizers. Little is known about denitrification N losses.
Methods
Soil denitrification rates were measured by the acetylene inhibition technique applied to anaerobically incubated soil samples. Four different greenhouse management systems were differentiated: Conventional flood irrigation and over-fertilization (CIF, 800 kg N ha−1, 460 mm); CIF plus straw incorporation (CIF+S, 889 kg N ha−1, 460 mm); Drip fertigation with reduced fertilizer application rates (DIF, 314 kg N ha−1, 190 mm); DIF plus straw incorporation (DIF+S, 403 kg N ha−1, 190 mm). Soil denitrification was measured on nine sampling dates during the growing season (Feb 2019-May 2019) for the top-/ subsoil (0 – 20/ 20- 40 cm) and on three sampling dates for deep soils (40-60/ 80-100 cm). Data was used to constrain N-input-output balances of the different vegetable production systems.
Results
Rates of denitrification were at least one magnitude higher in topsoil than in sub- and deep soils. Total seasonal denitrification N losses for the 0 – 40 cm soil layer ranged from 76 (DIF) to 422 kg N ha−1 (CIF+S). Straw addition stimulated soil denitrification in top- and subsoil, but not in deep soil layers. Integrating our denitrification data (0-100 cm) with additional data on N leaching, N2O emissions, plant N uptake, and NH3 volatilization showed, that on average 50 % of added N fertilizers are lost due to denitrification.
Conclusions
Denitrification is likely the dominant environmental N loss pathway in greenhouse vegetable production systems. Reducing irrigation and fertilizer application rates while incorporating straw in soils allows the reduction of accumulated nitrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the vegetables produced in China, about 30 % are grown in intensive greenhouse production systems (Hu et al. 2017), which currently cover 4.67 million ha (Fei et al. 2018) and are expanding at an annual growth rate of 10 % (Guo et al. 2012a, b). Farmers typically apply nitrogen (N) fertilizer (synthetic and manure) in two cropping seasons per year at rates that exceed plant requirements by a factor of 4 – 5, amounting to >2000 kg N ha−1 yr−1 (Fan et al. 2014; Yang et al. 2016). Moreover, traditional flood irrigation is usually used, which is up to 1200-1600 mm per year and is 2-3 times higher than plant demand (Lv et al. 2019). This practice promotes high N leaching losses, resulting in the contamination of groundwater with nitrate (Lv et al. 2019; Qasim et al. 2021; Zhu et al. 2005), and leading to major health risks for the local population. In the vegetable production areas of Shandong province, China, 70-80 % of well water taken from as deep as 110 m exceeded the NO3− drinking water standard (above 10 mg N L−1) (Lin et al. 2011; Zhu et al. 2005). The conventional farmer practices drive not only high nitrate leaching but also high N losses from soils in the gaseous phase, partially in the form of the greenhouse gas nitrous oxide (N2O) (Qasim et al. 2021; Yao et al. 2019; Zhao et al. 2021a, b). To mitigate the environmental impacts, alternative fertilizer and water management practices have been proposed, including the reduction of N application and irrigation rates and/or the use of drip fertigation schemes, which have been shown to reduce environmental N losses from greenhouse vegetable systems (Liang et al. 2020; Yao et al. 2019).
In addition to the unsustainable use of high amounts of fertilizer and water resources, greenhouse vegetable production is challenged by low soil organic carbon (SOC) content, which is created partially due to the way the greenhouses are typically constructed. This involves the removal of the topsoil, which is then used as the north-facing back wall of the greenhouse, which allows winter vegetable production without heating. Topsoil removal leaves the subsoil exposed, which is naturally depleted in SOC. Low SOC content not only negatively affects soil nutrient retention but also results in low soil respiration rates (Liu et al. 2014), which causes greenhouse CO2 concentrations in the midday to drop below atmospheric concentrations and ultimately limiting crop production (Wang et al. 2019; Wittwer 1990). Thus, approaches to improve the sustainability of vegetable production in greenhouse systems aim to reduce fertilization rates and water demand and to increase the soil SOC content. This has led to research in switching management practices from flood to drip irrigation (and fertigation) and to the investigation of straw and biochar application to improve SOC. (Lv et al. 2019; Zhao et al. 2020).
Denitrification is one of the most important microbial-loss pathways that may ameliorate NO3− leaching losses from greenhouse gas production (Wang et al. 2019). Denitrification is a heterotrophic, anaerobic microbial process in which nitrogen (N) oxides, nitrate (NO3−), and nitrite (NO2) are reduced to dinitrogen (N2) via intermediate gaseous phases, nitric oxide (NO), and nitrous oxide (N2O) (Butterbach-Bahl et al. 2013). Thus, the magnitude of denitrification in soil layers depends on anaerobic conditions, NO3− supply, and a source of easily degradable carbon substrates (McCarty and Bremner 1992). In agricultural soils, denitrification may remove several hundred kilograms of nitrate (NO3−), with the highest rates in irrigated, nitrogen-fertilized soils (Barton et al. 1999), as in those soils a switch between aerobic and anaerobic conditions frequently occur, promoting a tight coupling of nitrification and denitrification activity and loss of mineral N by denitrification; however, the application of straw or other organic matter in soils may further stimulate N losses through denitrification (Wu et al. 2018).
Denitrification in the soil layers of greenhouse vegetable production is rarely studied. Earlier research indicates that denitrification mainly occurs in the topsoil horizon (van Cleemput 1998), although significant denitrification activity has been found in other sub-surface agricultural systems with high N inputs, especially where organic C availability is adequate (and in some cases added as part of management), for example in intensive farmland of North China Plain (Yuan et al. 2019), a cereal crop field in Japan (Kamewada 2007), and in an intensively managed pasture in Ireland (Jahangir et al. 2012). In the greenhouse systems included in this study, Zhou et al. (2010) speculated that due to high irrigation and fertilization and increased NO3− availability in subsoils, high denitrification rates might occur in subsoil layers, but experimental evidence was not provided. Moreover, flood irrigation, i.e. the common irrigation practice in intensive greenhouse vegetable production, results not only in high amounts of nitrate leaching but also in significant leaching losses of both dissolved organic carbon and nitrogen (DOC and DON) (Luo et al. 1998; Lv et al. 2019). DON and DOC may serve as an energy source for denitrification in subsoils, which would also explain observations about its importance as a mechanism for the removal of excess NO3− (Bouwman et al. 2013; Fenton et al. 2009; Sotomayor and Rice 1996).
Methods for measuring rates of denitrification in soils have been reviewed e.g. by Groffman et al. (2006). More recently, the work of Amaraz et al. (2020) re-evaluated the strength and weaknesses of the different techniques available, i.e. the acetylene inhibition technique, the helium gas flow technique, 15 N-NO3 tracing, 15 N-N2O pool dilution approaches, measurements of N2:Ar ratios, or quantification of clumped isotopes of N2. As the acetylene inhibition technique allows high troughput of samples at low costs and is relatively simple to learn, Almaraz et al. (2020) recommends the use of this technique for comparing “instantaneous fluxes among sites or experimental treatments”. The acetylene inhibition technique builds the basis for the denitrification enzyme assay (DEA). DEA is commonly used to determine the highest possible rates of denitrification (i.e. the potential) and as well as to determine the limiting substrates of soil denitrification Freschet et al. 2008; Groffman et al. 1999; Menendez et al. 2008). The DEA uses the acetylene block, in which soil samples are homogenized and placed in an anaerobic environment with the presence of acetylene, which blocks the production of the final product, N2, and allows the accumulation of N2O, which can be easily measured on a gas chromatograph (Groffman et al. 1999). This method is considered a maximum potential rate and not an actual rate because the assay is conducted using soil slurries in ideal conditions, which include the addition of substrates (NO3 and labile organic carbon) and an anaerobic environment. Therefore, the use of acetylene methods in calculating N balance should be treated with caution. Using the results from DEAs with no additional substrates added (i.e. with only the available substrates present in the original sample) may help to better represent actual rates, though the acetylene also blocks nitrification and the possibility of coupled nitrification-denitrification, which would underestimate actual rates (Groffman et al. 2006, Amaraz et al., 2020). Despite the well-documented drawbacks of this method, it is also the most common in ecosystem studies aiming to understand controls of denitrification (Almaraz et al. 2020), and has been used in other studies to estimate N losses due to denitrification, even sometimes underestimating losses compared to other techniques (e.g. Lin et al. 2021). This method further has the advantage that a large number of samples can be analyzed inexpensively, which allows understanding of seasonal dynamics and reduces uncertainty; and it also has the ability to assess controls such as limitation by NO3 or organic C (Groffman et al. 2006).
Understanding the role of denitrification in overall N losses and in the context of a nitrogen balance for intensive greenhouse systems under different management schemes (i.e. drip versus flood irrigation, with or without straw incorporation) is critical for sustainable management; yet, there is no study available that we know of that quantifies this. Using an N-balance approach, Ti et al. (2015) estimated that 1/3 of the applied N fertilizer in these greenhouse vegetable systems in China may be lost as gaseous N; however, no denitrification measurements were made. Here we use DEA to determine potential denitrification rates (DR) and its controls along with the soil profile to: (1) Quantify the effects of irrigation schemes and straw application on denitrification in different soil depths; (2) Determine whether NO3− or C limits denitrification along with the soil profile; (3) Identify growing season variation and quantify seasonal losses of N estimated by denitrification; and (4) Use estimates of N losses via denitrification to assess its possible role in closing the N balance in the investigated greenhouse vegetable systems. We hypothesized that the highest denitrification rates would occur at the beginning of the cropping season following basal fertilization with chicken manure and synthetic N fertilizer, as easily degradable C and N substrates are added, while the plant N uptake is still low. Moreover, we hypothesized that denitrification would be higher in soil treatments with flood irrigation and straw addition, as this management practice is associated with higher soil moisture values and additional C substrates for microbial activity are provided. Finally, we hypothesized that denitrification would be mainly limited by C rather than NO3−, given that high rates of N fertilization in greenhouse soils and that denitrification activity strongly declines with soil depth.
Materials and methods
Site description
Measurements of seasonal changes in denitrification were carried out at the field station of the Tianjin Academy of Agricultural Sciences, Wuqing District of Tianjin City, China (N 39°25’35”, E 116°57’18”) during the winter-spring tomato production season, from February to May 2019. The site is located in the northern temperate monsoon climate zone, with a 30 year (1987-2017) annual mean air temperature of 12.5 °C, annual mean precipitation of 518 mm, and annual mean sunshine hours of 2392 (Meteorological Observation Station, Wuqing). The solar greenhouse (dimensions: length of 65 m, width of 8 m, and height in the range of 1 to 4 m) was built in 2011 and has been used since then for tomato production in two seasons per year. Soils in this region are calcaric gley-soils with a silty loam texture. Characteristics of the soil in the greenhouse down to 1 m soil depth are given in Table S1.
Experimental design
Denitrification rates were measured in soils of five treatments, which were replicated three times using a randomized split-plot design. The treatments were:
-
(1)
Conventional flooding irrigation with over-fertilization (CIF). This represents the local farmers’ practice;
-
(2)
CIF + 8 t ha−1 (or 3.5 t C ha−1) of maize straw (CIF+S). Local farmers’ practice with straw added to maintain or increase soil organic carbon stocks;
-
(3)
Drip irrigation with reduced fertilization (DIF), representing recommended management to reduce irrigation water and fertilizer use;
-
(4)
DIF + 8 t ha−1 (or 3.5 t C ha−1) of maize straw (DIF+S);
-
(5)
DIF + 3.8 t ha−1 (or 2.1 t C ha−1) biochar (DIF+B), long-term treatment to study whether the addition of biochar is suitable to increase soil carbon concentrations.
Field preparation occurred on February 26, 2019, before the transplant of tomato seedlings and consisted of preparing the treatment plots and applying basal fertilizer. Each replicated plot consists of a raised bed (0.7 m in width) with a walk-way (width: 0.5 m) in between. For CIF treatments, a small furrow (width: 0.2 m, depth: 0.15 m) was dug in the middle of each raised bed to allow for flood irrigation. Field preparation also included the incorporation of basal fertilizer [all 200 kg N chicken manure plus (1.45 % N and 13.2 % C) 350 kg N chemical fertilizer (N-P2O5-K2O, 17-17-17) for CIF treatments] and crushed maize straw (2-4 cm) or biochar in the +S and +B treatments into the top 20 cm of the soil by a rotary machine (Table 1).
On the day of transplanting, CIF treatments were flood irrigated with 94 mm, and then subsequently flood irrigated every 10-15 days with 61 mm irrigation water with enough chemical fertilizer dissolved in the water to apply ~ 50 kg N ha−1 (a practice called fertigation) (Table S2). Irrigation water in DIF treatments was provided by drip emitters, which were spaced at 0.4 m intervals. DIF treatments were irrigated with 49 mm on the day of transplanting and then irrigation frequency was 1-3 days with 3-5 mm irrigation water, supplemented with 2-3 kg N ha−1 fertilizer, with amounts slightly varying depending on crop growth stage and weather. The total fertilizer rate was 800, 889, 314, 403, and 356 kg N ha−1 and irrigation amount was 460, 460, 190, 190, and 190 mm for CIF, CIF+S, DIF, DIF+S, and DIF+B, respectively. Details of fertilizer and water application are provided in Table 1 and Table S2.
Soil sample preparation and measurements
In all treatments, soil temperature (°C) and moisture (volume %) were continuously measured at 12 cm depth using a smart TDR sensor (Model: CS655, Campbell Scientific). Water-filled pore space (WFPS) was calculated from the volumetric soil moisture measurements using a standard equation described in Liu et al. (2013).
Soil samples for denitrification enzyme assays were collected for 0 – 20 cm and 20 – 40 cm depths after each irritation and fertilization event, which was approximately every 1-2 weeks, starting from 13 days after transplanting (details dates of soil sampling presented in Table S2). Additional bioassays were conducted for the 40-60 cm and 80-100 cm soil layers three times during the growing season: (a) at the start (13 days after tomato seedling transplanting (March 10, 2019)); (b) mid-season (day 52, April 18, 2019); and (c) at the end of the season (day 83, May 19, 2019). Soil samples were taken with a 4-cm soil auger. The soil samples were sieved using 5 mm mesh and stored at 4 °C in plastic bags before further processing, which was no longer than 4 days after sampling. Inorganic N concentrations were also measured in the 0-20 cm, 40-60 cm, and 80-100 cm soil layers three times during the growing season.
Soil moisture content was determined gravimetrically by oven drying samples at 105 °C for 24 h. For soil ammonium (NH4+) and nitrate (NO3−) analysis, 12 g fresh soil subsamples were sieved at 2 mm mesh size and extracted with 0.01 M CaCl2 at a 1:10 (w/w) soil to solution ratio and shaken for 1 h at 300 rpm at 25 °C. The extracts were filtered with a medium-speed qualitative filters (pore size 11 μm; Tongyongdianqi, China) and stored at -20 °C until analysis (Fan et al. 2014). The filtrate was analyzed for NH4+ and NO3− with a continuous flow analyzer, based on colorimetric chemistry (AA3, Bran & Luebbe, Nordstadt Hamburg, Germany).
Denitrification enzyme assay and calculation of cumulative denitrification rates
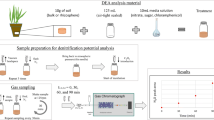
Rates of denitrification were measured using the denitrification enzyme assay (DEA) (Fig. 1), which is based on acetylene inhibition of the production of N2, as described by Groffman et al. (1999, 2006) and following a similar protocol to Malique et al. (2019). The incubations were done in an anaerobic environment with and without additions of media containing substrates (NO3 and a labile source of organic carbon (glucose)). The addition of chloramphenicol, which prevents microbial growth during the course of the incubation, was used, so the assay is considered a measure of the activity of the enzyme present in the sample (Tiedje et al. 1989; Groffman et al. 1999, 2006). DEA media solutions were prepared one day before the experiments and stored in a refrigerator at 4°C. To assess possible C or N limitation of denitrification, four solutions were prepared: i) control (CK), (no substrate addition); ii) NO3 only (0.72 g L−1 KNO3 or 0.1 g N kg−1 ds−1 ); iii) C only (0.5 g L−1 glucose or 0.2 g C kg−1 ds−1); iv) NO3 + C (0.1 g N kg−1 ds−1 + 0.2 g C kg−1 ds−1). Chloramphenicol was added (0.04 g L−1 or 0.1 g kg−1 ds−1) to all solutions. About 12.5 grams of fresh soil (equivalent to ~10 g dry weight) and 10 ml media were added into 125 ml flasks. The flasks were made gastight with butyl stoppers, and made anaerobic by alternating three times between evacuation and flushing with 99.99 % N2 gas, each for about 30 seconds. After that, flasks were brought to atmospheric pressure by inserting a needle briefly before adding 10.2 ml of 10 % acetylene (C2H2) to the headspace. Flasks were transferred to a rotary shaker (125 rpm) and incubated at 25°C for a total of 90 min. Gas samples (10 ml) were collected at 30 and 90 minutes in airtight syringes and replaced with an equal volume of N2 to avoid pressure changes. Gas samples were immediately analyzed for N2O by gas chromatography with a gas chromatograph (GC-14A, SHIMADZU) equipped with an Electron Capture Detector (ECD) and a Hayesep Q column (3 m, 1/8”), using N2 at 30 ml min−1 as a carrier gas, and a mixture of 5 % CO2 in N2 as make-up gas. The oven temperature was 40 °C, and the detector temperature was 340 °C.
Denitrification rates were calculated based on the changes in headspace N2O concentration (ppmv) between 30 and 90 min. The N2O dissolved in the liquid phase was calculated using the Bunsen coefficient (0.632) (Groffman et al. 1999). All measured concentrations were converted to mass units by applying the ideal gas law (Holland et al. 1999).
Where: Denitrification rate (ng N g−1 h−1 dry soil) was calculated as the rate of N2O production between 30 and 90 min, accounting for both gaseous and aqueous phases.
We calculated cumulative denitrification for the growing season (83 days) in units of kg N ha−1by first converting denitrification rates from the control treatment (no added C or N) to areal rates using bulk density and soil depth (including 0 – 100 cm layer), and calculating daily rates assuming that denitrification was constant over a 24 h period. We used the DR measured from the control (CK) treatment (no substrates added) rather than the potential denitrification rates (the +C+N treatment) to provide a more realistic estimate of actual denitrification rates – i.e. the maximum rate that could possibly be achieved with the levels of C and N present in the soil if anaerobic conditions were present. Then, we used linear interpolation to estimate the denitrification rates for non-sampling days for 83 days of the growing season (i.e. Feb – May, total 83 days). Since the first measurements were made 13 days after transplanting, we assumed that the denitrification rate on day zero was equal to the lowest measurements we made in the whole season. This assumption is reasonable because there is no irrigation or fertilization in the fallow period (6 weeks) prior to transplanting and the soils are therefore dry; however, we likely missed measurements of high rates of denitrification that may have occurred within the first 13 days due to basal fertilization (and irrigation) which occur at the same time as transplanting. Thus, we averaged the lowest measured rate and the rate measured on day 13 and assumed this rate over the initial 13 days in our calculation. We used error propagation to calculate the overall uncertainties of cumulative denitrification rates.
Calculation of N loss pathways
Gaseous losses of N2O, N2, and NH3
We estimated N2O losses from the plots by using daily or every other-day measurements of N2O fluxes measured from the study plots, which were done using a static chamber technique and presented in Zhao et al. (2021a). We used the cumulative N2O emissions data for 83 days of the growing season (i.e. Feb – May 2019). These data were interpolated in a manner similar to denitrification rates as described above, and the cumulative losses for the growing season were calculated. As these chamber measurements account for N2O, we also needed to estimate losses of N2. For this, we assumed that the denitrification measured with the use of acetylene inhibition represented the sum of N2O + N2 losses. We, therefore, subtracted the chamber-based N2O fluxes measured from the cumulative denitrification rates and assumed this represented N2 emissions. We are aware that the denitrification measurements via acetylene inhibition reflect the upper limit of actual denitrification in soils. We use this estimate together with other N loss pathways (crop N uptake, N leaching and soil N2O emissions) to evaluate whether denitrification can, within error margins, close the N budget in these intensively managed greenhouse vegetable production systems.
NH3 loss from volatilization was calculated from the mean of 19 observations from five studies from intensive greenhouse vegetable production in China Guo et al. 2012a, b; Hao et al. 2012; Ju et al. 2011; Li et al. 2011; Ti et al. 2015). From these studies, the percentage of applied fertilizer due to NH3 volatilization ranged from 0.02 to 2.66 %, with a mean value of 0.8 ± 0.2 %. We used this mean value applied to each treatment over the 83 days growing season for the N balance.
N Leaching losses
Leaching losses for the study plots and the same growing season were measured and presented by Zhao et al. (2021a). Briefly, leaching losses were estimated by calculating soil water percolation rates based on tensiometer readings and determining inorganic and organic N concentrations in soil water at 90 cm depth. For this, suction cups were installed and sampled daily for 3 d following each flood irrigation event in the CIF treatments, and the same sampling days were also used for DIF treatments. Details on the suction cup installation and leaching losses methods are reported in Lv et al. (2019).
Plant N uptake
Data on plant N uptake was taken from Lv et al. (2019), as this work was carried out in the same greenhouse and based on the same experimental design. Briefly, tomato yield from approximately 50 plants (1 row of plants) from each plot was measured throughout the season and subsampled for N content. Samples were oven-dried at 75 °C for 48 h. Samples were ground in a knife mill and then placed in a ball mill (MM200, Germany) for preparation for analysis for total N by an elemental analyzer (Costech ECH 4024, Italy). In addition, at the end of the harvest, 3 number of plants from each plot (including roots, stem, leaves) were sampled to determine their N content.
Statistical analysis
A two-way analysis of variance (ANOVA) was performed followed by Bonferroni’s multiple comparison test to assess quantitative differences in denitrification between management practices (plot treatments), soil depths, sampling time, and substrate additions, with p <0.05 considered to be significant. Data were presented as mean ± standard error of the mean. Pearson correlation was used to investigate correlation between denitrification rates with soil nitrate concentration and soil moisture content. All statistical analyses were performed using Prism 5 software (https://graphpad-prism.software.informer.com/5.0/).
Results
Soil environmental conditions
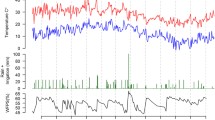
Soil temperature in all treatments ranged from 11 °C at the beginning to 23 °C towards the end of the growing season (mean: 17.6 ± 0.9 °C). Soil moisture ranged from 39 to 76 % in CIF treatments (mean: 61 ± 3 % WFPS) and for DIF treatments from 49 to 69 % (mean: 59 ± 3 % WFPS, Fig. 2). While soil moisture was relatively stable in DIF treatments, it varied greatly with flood irrigation events in CIF treatments.
Observed changes in soil moisture (measured as %WFPS, solid lines) and soil temperature (dashed lines) for the CIFs (Average of CIF and CIF+S) and DIFs (Average of DIF, DIF+S and DIF+B) treatments. The black arrow shows the initial flood irrigation on the day of transplanting of tomato seedlings. Blue arrows indicate days of flood irrigation for CIFs treatments, while “+” indicates that fertilizer was added to the irrigation water (top dressing). DIFs treatments were fertigated (irrigation water + fertilizer) every 2-3 days during the growing season
Extractable nitrate (NO3−) and ammonium (NH4+) soil concentrations in the field treatments, soil depths, and sampling dates are shown in (Fig. S2). For all three sampling dates, soil NO3− concentrations in the topsoil (0-20 cm) were >100 mg N kg−1 dry soil (ds); however, in deep soil, i.e. in the 40-60 cm and 80-100 cm layers, soil NO3− concentrations were mostly <100 mg N kg−1 ds. Overall, the lowest values most commonly occurred for the straw incorporated field treatments i.e. CIF+S and DIF+S. Soil NH4+ concentrations in the topsoil as well as in the deep soil were at least one magnitude lower (<10 mg N kg−1 ds) than soil NO3− concentrations and dropped to <5 mg N kg−1 ds for both samplings dates at mid and end of the season. In most cases, there were no significant effects of management practice nor soil depth effect on soil NH4+ concentrations.
Seasonality of denitrification rates
Rates of denitrification (DR) as measured in control (CK, no substrate added) varied largely (0.1 to 5.7 mg N kg−1 d−1) across the tomato cropping season in the topsoil (0-20 cm) as well as in the subsoil (20-40 cm) (Fig. 3). In topsoil, the highest DR were observed in the first month of the cropping season, i.e. with juvenile tomato plants. During this period, DR in the topsoil reached up to 4.7-5.7 mg N kg−1 d−1 for the treatments with straw addition (CIF+S and DIF+S), while for the treatments without straw additions, maximum DR reached up to 4.4 mg N kg−1 d−1. DR in the subsoil were significantly lower for all plot treatments during the first month, though maximum rates were still observed for the straw treatments with values up to 1.0 mg N kg−1 d−1 (Fig. 3). Across all treatments and for both soil layers, (i.e. for 0-20 cm and 20-40 cm), DR declined towards the end of the cropping season, only a maximum of 1.2 mg N kg−1 d−1 in 0-20 cm soil depth and <0.2 mg N kg−1 d−1 in 20-40 cm soil depth (Fig. 3).
Seasonality and cumulative denitrification rates (CK:water addition only) as observed in topsoil (0-20 cm) and subsoil (20-40 cm) for different treatments (CIF;CIF+S;DIF;DIF+S;DIF+B) during tomato growing period. Given are means ± SE of three replicates. Blue arrows indicate days of flood irrigation for CIFs treatments, while “+” indicates that fertilizer was added to the irrigation water (top dressing). DIFs treatments were fertigated (irrigation water + fertilizer) every 2-3 days during the growing season
The highest mean cumulative denitrification from the topsoil and subsoil were observed for the CIF+S treatment (368 ±117 kg N ha−1 and 54 ± 16 kg N ha−1 respectively) (Table 2) while the lowest rates were observed for the DIF treatment (65 ± 18 kg N ha−1for topsoil and 12 ± 2 kg N ha−1 for the subsoil) (Table 2). Overall, cumulative denitrification for the first 40 cm of soil, i.e. topsoil plus subsoil, declined in the following order: CIF+S > CIF ≥ DIF+S > DIF+B ≥ DIF (“>” indicates significant differences at p<0.05 while ≥ indicates not significant).
Rates of denitrification in the deep soil layers, i.e. in 40-60 cm and 80-100 cm soil depth, were measured three times: at the beginning (day 13), in the middle (day 52), and at the end (day 83) of the growing period (Fig. 4). Compared to mid-season, denitrification was generally higher towards the end of the season for both deep soil layers, with denitrification rates being, in most cases, not different between treatments. The maximum denitrification rate in the 40-60 cm soil layer reached up to 0.07 mg N kg−1 d−1, while in the 80-100 cm soil layer, the maximum was 0.28 mg N kg−1 d−1, observed at the start of the season (Fig. 4).
Observed rates of denitrification in three different soil depths (x-axis: 0-20, 40-60 and 80-100 cm), at three sampling dates (columns: beginning, middle and end of growing season), and for the various treatments investigated (rows: CIF, CIF+S, DIF, DIF+S, DIF+B). Shown are means (±SE) of three replicates. Different small letter superscripts indicate significant differences (P<0.05) of denitrification rates between the substrates addition for a given sampling date, soil depth and treatment. Different capital letter superscripts indicate significant differences (P<0.05) of denitrification rates between different soil depths for a given substrates, sampling date and treatment
C and N limitation of soil denitrification rates
Generally, the addition of NO3− to soil samples taken from the different plot treatments and different soil depths did not stimulate denitrification compared to controls (Fig. 5; Table 2), with the exception of subsoil from CIF+S and DIF+S treatments, indicating that denitrification is likely NO3− limited in these plots. However, adding glucose as a carbon source significantly (p<0.05) stimulated DR in the topsoil of all plot treatments (0-20 cm) by 124 - 416 %. This was not statistically significant in the sub- or deep soil layers (Fig. 5), though there was a clear tendency that glucose additions stimulated denitrification. NO3− + C did not result in significant stimulation of potential denitrification rates compared to only glucose-C addition, except for in the straw addition treatments for both CIF+S and DIF+S in the top and subsoil layer (Fig. 5).
Percent stimulation rates of denitrification due to the addition of either NO3-, Glucose-C or NO3- plus Glucose-C to anaerobic soil samples in comparison to water additions only (DEA approach). Shown are results for the different treatments as mean ± SE values for nine (topsoil, 0-20 cm; subsoil, 20-40 cm) or three (deep soil layers 40-60 cm and 80-100 cm) sampling dates and three replicates per soil layer and sampling date. Different capital letter superscripts indicate significant differences (P<0.05) of denitrification rates between various substrate additions. Different small letter superscripts indicate significant differences (P<0.05) of denitrification rates between treatments for a given substrate. * indicate significant difference of denitrification rates as compared to the control (water addition only)
Contribution of denitrification N losses to the total N balance
Combining measurements across the soil profile from 0 to 100 cm, total seasonal denitrification for the 83 days growing season, N losses ranged from 113 to 478 kg N ha−1 across all treatments (Table 3). The highest cumulative denitrification N losses were calculated for the treatments with straw addition, i.e. CIF+S and DIF+S, whereas the lowest losses occurred for drip irrigation with reduced fertilization (DIF). Total seasonal N losses over the 83 days growing season in the form of soil N2O emissions ranged from 1.7 to 27.4 kg N ha−1 (Table 3, Zhao et al. 2021a). Thus, in relation to total N fertilization rates (314-889 kg N ha−1), soil emissions of N2O were marginal (0.4 – 3.1 % of applied N), while losses due to denitrification (N2O+N2) appear to account for 36 - 80 % of applied N (Table 3).
Figure 6 shows the results of the N balance calculations for the different plot treatments excluding the biochar treatment as we did not have data for N leaching losses and N plant uptake. For the treatments CIF, CIF+S, and DIF, N losses equaled fertilizer input within error margins, while for the DIF+S treatment, N outputs were most strongly, though not significantly, negative, suggesting reductions of soil N stocks. It should be noted that plant N uptake in both CIF treatments accounted for only about 19-21 % of the N input, while for the DIF treatments, the N uptake percentage was in the range of 36-44 %. Moreover, N2 losses due to denitrification were, in all cases, higher as N leaching losses (Fig. 6).
Nitrogen input and output balance for different treatments of the investigated greenhouse tomato production system. Denitrification N2 losses were calculated for 1 m soil depth based on the DEA approach, with water addition only (control). From these values measured cumulative soil N2O emissions (Zhao et al. 2021a) were subtracted. Cumulative leaching losses in form of NO3− and DON were provided by Zhao et al. Both, soil N2O emissions, as well as nitrogen leaching losses, were measured in the frame of the same experiment. Values for plant N uptake were taken from Lv et al. (2019) with same experimental design, while estimates on soil NH3 volatilization were based on a literature review (see Methods). Given are mean values ± SE
Discussion
In our study we used the acetylene inhibition technique, which builds as well the basis of the denitrification enzyme assay (DEA) (Groffman et al. 1999), to quantify actual and potential denitrification rates in greenhouse soils. While the technique has known drawbacks such as e.g. a tendency to underestimate actual rates of denitrification (Groffman et al., 1999), it also has its strengths compared to other methods such as the helium gas flow technique (Butterbach-Bahl et al. 2002) or the 15 N-NO3 tracing (e.g. Weier et al. 1993a, b), as it allows to handle and run a high number of replicates, which is needed to compare instantaneous fluxes among sites or experimental treatments.
In this study, we used the DEA approach to quantify the growing season variation of denitrification in different soil depths and how they are affected by irrigation management (drip or flood), straw, and fertilizer management in intensive greenhouse vegetable production systems. The DEA approach measures potential denitrification rates and allows us to assess whether denitrification is C, NO3, or co-limited by C and NO3−. As incubations are run under strictly anaerobic conditions, this study’s measured denitrification rates probably represent the upper threshold of actual denitrification rates expected in situ. This is specifically true as soil moisture values in the topsoil (0-20 cm) in both water management systems, i.e. conventional flood irrigation (CIFs treatments) as well as in the drip irrigation (DIFs) treatments, were on average ~60 % WFPS, i.e. at the threshold at which denitrification starts to kick in as a respiratory pathway (Davidson et al. 2000). Despite the uncertainties, the DEA method has been widely used in studies aiming at quantifying denitrification rates and losses from agricultural and natural systems (Almaraz et al. 2020; Barton et al. 1999; Groffman et al. 2006; Lin et al. 2021).
Effects of water, fertilizer and straw management on soil denitrification
Our study supports our hypothesis that interactive effects of water, fertilizer, and straw management on soil denitrification exist and that DR are highest at the start of the growing season following basal fertilization and at a period of time with low crop N uptake. Across all treatments, denitrification activity during the first month of the growing season equaled 60-77 % of the cumulative seasonal denitrification. The seasonal decline in denitrification activity was most pronounced for the topsoil, while hardly any seasonal changes in deep soil denitrification were found. Overall, topsoil DR were at least one magnitude higher than rates observed for subsoil and deep soil layers (Table 2; Fig. 4), a difference which also has been noted before in other studies (Jahangir et al. 2012; Zhou et al. 2010), and in a case study investigating DR in soils of intensively farmed fields in the North China Plain (Haijing et al. 2019). Jahangir et al. (2012) also reported significant DR in subsoils, which was confirmed by our study as well. Despite the lower rates compared to topsoil rates, deep soil denitrification still contributed substantially (5-14 %) to the total denitrification. We also found that DR were significantly lower in soils irrigated by drip irrigation than by those irrigated by flood irrigation, and the incorporation of straw significantly stimulated topsoil denitrification (Table 2; Fig. 4). However, it is not possible to disentangle the effects of water and fertilizer management as both water and fertilizer were mixed before application with approximately two times higher total N application rates for the CIF treatments than the DIF treatments (Table 1). With regard to N additions, it is well established that denitrification generally increases in soils receiving higher application rates of nitrogen fertilizer and irrigation water, as it results in higher soil nitrate concentrations not only in topsoil but also in deep soil and thus, increases of substrate availability for denitrifiers (Burton et al. 2008; Lin et al. 2017; Yao et al. 2020). Our study also found a positive correlation between DR and soil NO3− (Fig. S3a). Irrigation is also well known to cause hot moments in denitrification as increased soil moisture content creates more anaerobic micro-sites, thereby initializing and stimulating denitrification activity (Barakat et al. 2016; Thomas et al. 2019; Vogeler et al. 2019; Zhang et al. 2018; see also Fig. S3b). Based on a literature review, Barakat et al. (2016) point out that flood irrigation enhances environmental N losses via leaching and denitrification, while drip irrigation “seems to be the technique with the least unwanted impacts”.
As we hypothesized, straw-amended treatments had a significantly positive effect on denitrification (cumulative DR in the top- and subsoils of the CIF+S increased by 39 % and 157 % respectively, and in the DIF+S treatment by 312 % and +8 % (Table 2; Fig. 3). The stimulating effect of straw incorporation on soil denitrification is well described in the literature and is likely due to three effects: (a) supply of extra C substrate for denitrification (Chen et al. 2013; Weier et al. 1993a, b; Wu et al. 2018); (b) increase of soil anaerobiosis as soil respiration is stimulated due to the breakdown of newly-added organic matter (Cleveland et al. 2007); and (c) additional supply of N substrate due to ammonification of organic matter (Chen et al. 2013), though N immobilization, leading to reduced availability, can also occur if the C:N ratio of the added straw is >40 (Yao et al. 2010; Yang et al. 2018). The addition of biochar (DIF+B treatment) did not significantly increase denitrification in top- or subsoil, most likely as biochar is rather resistant to decomposition (Lehmann et al. 2009), especially as here we used biochar obtained via pyrolysis of parent vegetable residues and processed at 450 °C which likely reduced the availability of labile organic carbon.
What is limiting soil denitrification? Carbon or NO3 − availability, or both?
The relative availability of carbon (C) and nitrate (NO3−) substrates in soil has a significant effect on total denitrification (Miller et al. 2008) and anaerobiosis is regarded as a primary control of denitrification (Andreae and Schimel 1989). Our experimental results clearly show that carbon substrate availability limited denitrification in the topsoil, as evidenced by the fact that additions of glucose-C stimulated denitrification by 121-416 % across all treatments. These results agree with our third hypothesis that denitrification in greenhouse vegetable soils are mainly limited by C. Similarly, Lei et al. (2010) and Ren et al. (2014) reported that average soil organic carbon (SOC) concentration in the greenhouse soils in Shouguang, Shandong province, China is <12 g kg−1, which is below the threshold of 20 g C kg−1 at which soil microbial microbiome is seriously degraded (Loveland and Webb 2003). Weier et al. (1993) also showed that easily decomposable C substrate increases the conversion of N2O to N2 in wet soils, which is consistent with our results showing high DR in the wetter soils (Table 2). The availability of sufficient C stimulates the flow of electrons driving denitrification, further promoting the last step of denitrification (Weymann et al. 2010). Chen et al. (2018) concluded that the low DR in deep soil should not be only attributed to low abundance of denitrifiers, but due to the low availability of substrate caused by low carbon availability. Most of the studies reported similar conclusions of C limitation in subsoil of different agriculture systems after incorporating C substrates or dissolved organic carbon and the addition of which could be a potential method to reduce nitrate leaching by enhancing subsoil denitrification (Jahangir et al. 2012; Qin et al. 2017).
In this study, generally, we didn’t observe a significant effect of NO3− addition on denitrification rates, which may be due to high NO3− concentrations already present in the soil. NO3 was limiting only for the straw addition treatment (in both the top and sub-soil). Senbayram et al. (2012) reported that a significant stimulation of denitrification by addition of NO3− may only occur when soil NO3− concentration is < 20 mg NO3−-N kg−1 dry soil, while in our study, topsoil NO3− concentrations were in all treatments >100 mg NO3−-N kg−1 dry soil due to fertilization rates well above >300 kg N ha−1 season−1.
Nitrogen balance under the greenhouse tomato production system
Our study is the first one presenting a full N balance based on measurements of soil denitrification for intensive vegetable production systems in China. Moreover, estimates of plant N uptake, nitrogen leaching losses, and soil N2O emissions are based on measurements carried in the frame of the same experiment (Lv et al. 2019; Zhao et al. 2021a). Measuring denitrification is rather difficult task in the field and previous studies used un-accounted for N in mass balance approaches to estimate N-losses via denitrification at 35-40 % (Castaldelli et al. 2020; Qasim et al. 2021; Ti et al. 2015). These estimates are slightly lower than our estimated N losses via denitrification, which was about 50 % (36 – 80 %; Table 3) across all treatments. This difference could be due to different soil management practices, especially the incorporation of straw stimulates DR in our study.
We are aware of the limitations of DEA with acetylene block approach, including that it may suppress microbial respiration (Zhang et al. 2009), and the inhibition of N2O reduction to N2 might be incomplete with acetylene (Jury et al. 1982). We also know that these denitrification measurements likely represent the upper limit of actual denitrification, though may also have the possibility to under-estimate denitrification due to the inhibition of coupled nitrification-denitrification (Almaraz et al. 2020; Lin et al. 2021). Even in view of all uncertainties associated with using DEA approach and scaling soil denitrification to N budget, our N balance shows that the measured DR are in a logical range and that such measurements are helpful to constrain the fate of added fertilizer N. We were rather surprised, that by considering measured DR we were able to close the N balance of CIF, CIF+S, and DIF treatments within standard error margins. Only for DIF+S, the N balance was negative (26.5 %), indicating that straw addition in combination with drip fertigation may result in a net removal of N from the soil N stocks. The applied N in DIF+S (403 kg N ha−1) with less irrigation (190 mm) remains for a longer time in the soil layers (0-100 cm) and the incorporation of straw helps to provide sufficient C to denitrifiers in converting NO3− to N2.
Conclusions
Our results show that N losses due to soil denitrification are at approximately the same magnitude as nitrogen leaching and that straw addition strongly stimulates denitrification. We even found some evidence that straw addition may result in the reduction of soil mineral N stocks. This is a rather positive message, given that the mineral nitrogen present in these soils still far exceeds plant demand which in some studies has already been shown to diminish crop yields due to N salinization effects (Qasim et al. 2021). Furthermore, straw also significantly reduces nitrogen leaching losses (Lv et al. 2019) which may not only be used to increase soil respiration and to increase soil C stocks but also for the restoration of greenhouse soils due to over-fertilization. While our study has arrived at similar conclusions as other studies, this work nonetheless points to the necessity of further developing alternative greenhouse management systems to evaluate the most optimal way to mitigate N loss pathways. More comprehensive studies with more reliable techniques are required to quantify seasonal and cumulative DR and constrain the contribution of denitrification N2 in the total N output budget in greenhouse vegetable systems.
Change history
13 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Almaraz M, Wong MY, Yang WH (2020) Looking back to look ahead: a vision for soil denitrification research. Ecology 101(1):e02917
Andreae MO, Schimel DS (1989) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Plant Growth Regul 10:383–384. https://doi.org/10.1007/BF00024600
Barakat M, Cheviron B, Angulo-Jaramillo R (2016) Influence of the irrigation technique and strategies on the nitrogen cycle and budget: A review. Agric Water Manag 178:225–238
Barton L, McLay CDA, Schipper LA, Smith CT (1999) Annual denitrification rates in agricultural and forest soils: a review. Soil Res 37(6):1073–1094
Bouwman L, Goldewijk KK, Van Der Hoek KW, Beusen AH, Van Vuuren DP, Willems J, Stehfest E (2013) Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc Natl Acad Sci U S A 110(52):20882-20887
Burton DL, Zebarth BJ, Gillam KM, MacLeod JA (2008) Effect of split application of fertilizer nitrogen on N2O emissions from potatoes. Can J Soil Sci 88(2):229–239
Butterbach-Bahl K, Willibald G, Papen H (2002) Soil core method for direct simultaneous determination of N 2 and N 2 O emissions from forest soils. Plant Soil 240(1):105–116
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc B: Biol Sci 368(1621):20130122
Castaldelli G, Vincenzi F, Fano EA, Soana E (2020) In search for the missing nitrogen: Closing the budget to assess the role of denitrification in agricultural watersheds. Appl Sci 10(6):2136
Chen H, Li X, Hu F, Shi W (2013) Soil nitrous oxide emissions following crop residue addition: a meta-analysis. Glob Chang Biol 19(10):2956–2964
Chen S, Wang F, Zhang Y, Qin S, Wei S, Wang S, Liu B (2018) Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ Microbiol 20(3):980–992
Cleveland CC, Nemergut DR, Schmidt SK, Townsend AR (2007) Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82(3):229–240
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides: using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils. Bioscience 50(8):667–680. https://doi.org/10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2
Fan Z, Lin S, Zhang X, Jiang Z, Yang K, Jian D, Wang J (2014) Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable production. Agric Water Manag 144:11–19
Fei C, Zhang SR, Liang B, Li JL, Jiang LH, Xu Y, Ding XD (2018) Characteristics and correlation analysis of soil microbial biomass phosphorus in greenhouse vegetable soil with different planting years. Acta Agric Bor-Sin 33(1):195–202
Fenton O, Richards KG, Kirwan L, Khalil MI, Healy MG (2009) Factors affecting nitrate distribution in shallow groundwater under a beef farm in South Eastern Ireland. J Environ Manag 90(10):3135–3146
Freschet GT, Masse D, Hien E, Sall S, Chotte JL (2008) Long-term changes in organic matter and microbial properties resulting from manuring practices in an arid cultivated soil in Burkina Faso. Agric Ecosyst Environ 123(1–3):175–184
Groffman PM, Holland EA, Myrold DD, Robertson GP, Zou X (1999) Denitrification. Standard soil methods for long-term ecological research, pp 272–288
Groffman PM, Altabet MA, Böhlke JK, Butterbach-Bahl K, David MB, Firestone MK, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16(6):2091–2122
Guo S, Sun J, Shu S, Lu X, Tian J, Wang J (2012a) Analysis of general situation, characteristics, existing problems and development trend of protected horticulture in China. China Vegetables 18:1–14
Guo Y, Li B, Di H, Zhang L, Gao Z (2012b) Effects of dicyandiamide (DCD) on nitrate leaching, gaseous emissions of ammonia and nitrous oxide in a greenhouse vegetable production system in northern China. Soil Sci Plant Nutr 58(5):647–658. https://doi.org/10.1080/00380768.2012.726921
Haijing YUAN, Shuping QIN, Wenxu DONG, Chunsheng HU, Manevski K, Xiaoxin LI (2019) Denitrification rate and controlling factors for accumulated nitrate in the deep subsoil of intensive farmlands: a case study in the North China Plain. Pedosphere 29(4):516–526
Hao XY, Gao W, Wang YJ, Jin JY, Huang SW, Tang JW, Zhang ZQ (2012) Effects of combined application of organic manure and chemical fertilizers on ammonia volatilization from greenhouse vegetable soil. Sci Agric Sin 45(21):4403–4414
Holland EA, Robertson GP, Greenberg J, Groffman PM, Boone RD, Gosz JR (1999) Soil CO2, N2 and CH4 exchange. Standard soil methods for long-term ecological research, pp 185–201
Hu W, Zhang Y, Huang B, Teng Y (2017) Soil environmental quality in greenhouse vegetable production systems in eastern China: current status and management strategies. Chemosphere 170:183–195
Jahangir MM, Khalil MI, Johnston P, Cardenas LM, Hatch DJ, Butler M, Richards KG (2012) Denitrification potential in subsoils: a mechanism to reduce nitrate leaching to groundwater, 147. Agric Ecosyst Environ :13–23
Ju M, Xu Z, Wie MS, Guang XX, Zhao LZ (2011) Nitrogen balance and loss in a greenhouse vegetable system in southeastern China. Pedosphere 21(4):464–472
Jury WA, Letey J, Collins T (1982) Analysis of chamber methods used for measuring nitrous oxide production in the field. Soil Sci Soc Am J 46(2):250–256
Kamewada K (2007) Vertical distribution of denitrification activity in an Andisol upland field and its relationship with dissolved organic carbon: Effect of long-term organic matter application. Soil Sci Plant Nutr 53(4):401–412
Lehmann J, Czimczik C, Laird D, Sohi S (2009) Stability of Biochar in the Soil. Biochar for Environmental Management: Science and Technology, pp 183
Lei B, Fan M, Chen Q, Six J, Zhang F (2010) Conversion of wheat–maize to vegetable cropping systems changes soil organic matter characteristics. Soil Sci Soc Am J 74(4):1320–1326
Li Y, Wu X, Mei X, Duan M, Wu Q, Wu H, Cai D (2011) Effects of nitrogen application on ammonia volatilization in greenhouse soil under condition of conventional irrigation. Transactions of the Chinese Society of Agric Enginee 27(7):23–30. https://doi.org/10.3969/j.issn.1002-6819.2011.07.005
Liang H, Lv H, Batchelor WD, Lian X, Wang Z, Lin S, Hu K (2020) Simulating nitrate and DON leaching to optimize water and N management practices for greenhouse vegetable production systems. Agric Water Manag 241:106377
Lin S, Liu MJ, Fan ZB (2011) Comprehensive management of water and nutrients in vegetable plants. In: Wang JG (ed) Restoration of Degraded Soil and Efficient Utilization of Resources in Protected Vegetable Fields. Publisher of China Agricultural University. 209-229. (In Chinese)
Lin W, Ding J, Li Y, Zheng Q, Zhuang S, Zhang D, Li Y (2021) Determination of N2O reduction to N2 from manure-amended soil based on isotopocule mapping and acetylene inhibition. Atmos Environ 244:117913
Lin Y, Ding W, Liu D, He T, Yoo G, Yuan J, Fan J (2017) Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol Biochem 113:89–98
Liu Q, Qin Y, Zou J, Guo Y, Gao Z (2013) Annual nitrous oxide emissions from open-air and greenhouse vegetable cropping systems in China. Plant soil 370(1–2):223–233
Liu C, Lu M, Cui J, Li B, Fang C (2014) Effects of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Glob Chang Biol 20(5):1366–1381
Loveland P, Webb J (2003) Is there a critical level of organic matter in the agricultural soils of temperate regions: a review. Soil Till Res 70:1–18
Luo J, Tillman RW, White RE, Ball PR (1998) Variation in denitrification activity with soil depth under pasture. Soil Biol Biochem 30(7):897–903
Lv H, Lin S, Wang Y, Lian X, Zhao Y, Li Y, Butterbach-Bahl K (2019) Drip fertigation significantly reduces nitrogen leaching in solar greenhouse vegetable production system. Environ Pollut 245:694–701
Malique F, Ke P, Boettcher J, Dannenmann M, Butterbach-Bahl K (2019) Plant and soil effects on denitrification potential in agricultural soils. Plant Soil 439(1–2):459–474
McCarty GW, Bremner JM (1992) Availability of organic carbon for denitrification of nitrate in subsoils. Biol Fertil Soils 14(3):219–222
Menéndez S, Lopez-Bellido RJ, Benítez-Vega J, Gonzalez-Murua C, Lopez-Bellido L, Estavillo JM (2008) Long-term effect of tillage, crop rotation and N fertilization to wheat on gaseous emissions under rainfed Mediterranean conditions. Eur J Agron 28(4):559–569
Miller MN, Zebarth B, Dandie CE, Burton DL, Goyer C, Trevors JT (2008) Crop residue influence on denitrification, N2O emissions and denitrifiers community abundance in soil. Soil Biol Biochem 40(10):2553–2562
Qasim W, Xia L, Shan L, Li W, Zhao Y, Butterbach-Bahl K (2021) Global greenhouse vegetable production systems are hotspots of soil N2O emissions and nitrogen leaching: a meta-analysis. Environ Pollut 272:116372. https://doi.org/10.1016/j.envpol.2020.116372
Qin S, Hu C, Clough TJ, Luo J, Oenema O, Zhou S (2017) Irrigation of DOC-rich liquid promotes potential denitrification rate and decreases N2O/(N2O+ N2) product ratio in a 0–2 m soil profile. Soil Biol Biochem 106:1–8
Ren T, Wang J, Chen Q, Zhang F, Lu S (2014) The effects of manure and nitrogen fertilizer applications on soil organic carbon and nitrogen in a high-input cropping system. PloS One 9(5):e97732
Senbayram M, Chen R, Budai A, Bakken L, Dittert K (2012) N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric Ecosyst Environ 147:4–12. https://doi.org/10.1016/j.agee.2011.06.022
Sotomayor D, Rice CW (1996) Denitrification in soil profiles beneath grassland and cultivated soils. Soil Sci Soc Am J 60(6):1822–1828
Thomas SM, Fraser PM, Hu W, Clough TJ, van der Klei G, Wilson S, Baird D (2019) Tillage, compaction and wetting effects on NO3, N2O and N2 losses. Soil Res 57(6):670–688
Ti C, Luo Y, Yan X (2015) Characteristics of nitrogen balance in open-air and greenhouse vegetable cropping systems of China. Environ Sci Pollut Res 22(23):18508–18518
Tiedje JM, Simkins S, Groffman PM (1989) Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. Plant Soil 115(2):261–284
Van Cleemput O (1998) Subsoils: chemo-and biological denitrification, N2O and N2 emissions. Nutr Cycl Agroecosyst 52(2–3):187–194
Vogeler I, Thomas S, van der Weerden T (2019) Effect of irrigation management on pasture yield and nitrogen losses. Agric Water Manag 216:60–69
Wang S, Liu W, Zhao S, Wang C, Zhuang L, Liu L, Zhu G (2019) Denitrification is the main microbial N loss pathway on the Qinghai-Tibet Plateau above an elevation of 5000 m. Sci Total Environ 696:133852
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci Soc Am J 57(1):66–72
Weier KL, Macrae IC, Myers RJK (1993) Denitrification in a clay soil under pasture and annual crop: estimation of potential losses using intact soil cores. Soil Biol Biochem 25(8):991–997
Weymann D, Geistlinger H, Well R, Von der Heide C, Flessa H (2010) Kinetics of N2O production and reduction in a nitrate-contaminated aquifer inferred from laboratory incubation experiments. Biogeosciences 7(6):1953–1972
Wittwer SH (1990) Implications of the greenhouse effect on crop productivity. HortSci 25(12):1560–1567
Wu D, Wei Z, Well R, Shan J, Yan X, Bol R, Senbayram M (2018) Straw amendment with nitrate-N decreased N2O/(N2O+ N2) ratio but increased soil N2O emission: A case study of direct soil-born N2 measurements. Soil Biol Biochem 127:301–304
Yang L, Huang B, Mao M, Yao L, Niedermann S, Hu W, Chen Y (2016) Sustainability assessment of greenhouse vegetable farming practices from environmental, economic, and socio-institutional perspectives in China. Environ Sci Pollut Res 23(17):17287–17297
Yang S, Wang Y, Liu R, Xing L, Yang Z (2018) Improved crop yield and reduced nitrate nitrogen leaching with straw return in a rice-wheat rotation of Ningxia irrigation district. Sci Rep 8(1):1–8
Yao Z, Zhou Z, Zheng X, Xie B, Mei B, Wang R, Zhu J (2010) Effects of organic matter incorporation on nitrous oxide emissions from rice-wheat rotation ecosystems in China. Plant Soil 327(1–2):315–330
Yao Z, Yan G, Wang R, Zheng X, Liu C, Butterbach-Bahl K (2019) Drip irrigation or reduced N-fertilizer rate can mitigate the high annual N2O+ NO fluxes from Chinese intensive greenhouse vegetable systems. Atmos Environ 212:183–193
Yao Z, Pelster DE, Liu C, Zheng X, Butterbach-Bahl K (2020) Soil N intensity as a measure to estimate annual N2O and NO fluxes from natural and managed ecosystems. Curr Opin Environ Sustain 47:1–6
Yuan H, Qin S, Dong W, Hu C, Manevski K, Li X (2019) Denitrification rate and controlling factors for accumulated nitrate in the deep subsoil of intensive farmlands: a case study in the North China Plain. Pedosphere 29(4):516–526
Zhang J, Li H, Wang Y, Deng J, Wang L (2018) Multiple-year nitrous oxide emissions from a greenhouse vegetable field in China: Effects of nitrogen management. Sci Total Environ 616:1139–1148
Zhang T, Xu X, Luo X, Han L, Wang Y, Pan G (2009) Effects of acetylene at low concentrations on nitrification, mineralization and microbial biomass nitrogen concentrations in forest soils. Chin Sci Bull 54(2):296–303
Zhao Y, Lin S, Liu Y, Li G, Wang J, Butterbach-Bahl K (2020) Application of mixed straw and biochar meets plant demand of carbon dioxide and increases soil carbon storage in sunken solar greenhouse vegetable production. Soil Use Manag 36:439–448
Zhao Y, Lv H, Qasim W, Wang Y, Lin S, Butterbach-Bahl K (2021a) Drip fertigation allows to significantly reduce environmental N losses in the form of N2O and nitrate while maintaining high yields of vegetable production in sunken solar greenhouses. Environ Pollut 273:11652
Zhao Y, Lin S, Wan L, Qasim W, Hu J, Xue T, Butterbach-Bahl K (2021b) Anaerobic soil disinfestation with incorporation of straw and manure significantly increases greenhouse gases emission and reduces nitrate leaching while increasing leaching of dissolved organic. N Sci Total Environ 785:147307. https://doi.org/10.1016/j.scitotenv.2021.147307
Zhou JB, Chen ZJ, Liu XJ, Zhai BN, Powlson DS (2010) Nitrate accumulation in soil profiles under seasonally open ‘sunlight greenhouses’ in northwest China and potential for leaching loss during summer fallow. Soil Use Manag 26(3):332–339. https://doi.org/10.1111/j.1475-2743.2010.00284.x
Zhu JH, Li XL, Christie P, Li JL (2005) Environmental implications of low nitrogen use efficiency in excessively fertilized hot pepper (Capsicum frutescens L.) cropping systems. Agric Ecosyst Environ 111(1–4):70–80. https://doi.org/10.1016/j.agee.2005.04.025
Acknowledgements
This work is part of the Sino-German cooperation project NIVEP (Minimizing nitrogen environmental impacts in intensive greenhouse vegetable production systems in Shandong, China) and was financially supported by the National Nature Sciences Foundation of China (grant no. 41761134087) and the German Science Foundation (Deutsche Forschungsgemeinschaft, DFG) (grant no. BU1173/19-1). We thank the Tianjin Academy of Agricultural Sciences for providing us with working and available facilities.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor:Zucong Cai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 736 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qasim, W., Zhao, Y., Wan, L. et al. The potential importance of soil denitrification as a major N loss pathway in intensive greenhouse vegetable production systems. Plant Soil 471, 157–174 (2022). https://doi.org/10.1007/s11104-021-05187-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05187-2