Abstract
Aims
In cold biomes, snow cover mitigates the harsh winter soil conditions, thereby enhancing overwinter decomposition of organic matter which controls the availability of nutrients for plants and microbial uptake at the beginning of the growing season. Yet, how this buffering effect is modulated by litter traits, soil characteristics and herbivory remains poorly studied.
Methods
We conducted a litter-bag experiment in four types of subalpine grasslands, two of which being naturally free of snow during most of the winter. Litter bags were filled either by grasses or forbs sampled in plots submitted the preceding summer to grasshopper grazing treatments.
Results
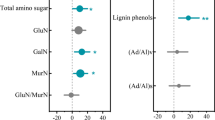
Snow cover strongly increased the decomposition of forbs, but not of grasses. Litter quality (low C:N and polyphenols triggering a priming effect) and soil ammonium content were correlated with litter decomposition rate in the presence of snow only, whereas soil organic matter content was positively associated with decomposition rate under both snow regimes. Herbivory did not affect decomposition.
Conclusions
Our findings may be explained by the functional differences between copiotrophic and oligotrophic microbes, copiotrophs being more sensitive to harsh abiotic conditions than oligotrophs. As long as copiotrophs are favored by high litter quality and nutrient-rich soils (high ammonium content), litter decomposition is enhanced in the absence of snow only.






Similar content being viewed by others
References
Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S (2010) A multi-trait approach reveals the structure and the relative importance of intra-vs. interspecific variability in plant traits. Funct Ecol 24:1192–1201
Allison SD (2005) Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol Lett 8:626–635
Allison SD, Lu L, Kent AG, Martiny AC (2014) Extracellular enzyme production and cheating in Pseudomonas fluorescens depend on diffusion rates. Front Microbiol 5:169
Baptist F, Yoccoz NG, Choler P (2010) Direct and indirect control by snow cover over decomposition in alpine tundra along a snowmelt gradient. Plant Soil 328:397–410
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bartoń K (2013) {MuMIn}: multi-model inference, {R} package version 1.9.13
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. ArXiv Prepr ArXiv14065823
Belovsky GE, Slade JB (2000) Insect herbivory accelerates nutrient cycling and increases plant production. Proc Natl Acad Sci 97:14412–14417. https://doi.org/10.1073/pnas.250483797
Bernard L, Foulquier A, Gallet C, Lavorel S, Clément JC (2019) Effects of snow pack reduction and drought on litter decomposition in subalpine grassland communities. Plant Soil 435:225–238
Bernard L, Decau M-L, Morvan-Bertrand A, Lavorel S, Clément JC (2020) Water-soluble carbohydrates in Patzkea paniculata (L.): a plant strategy to tolerate snowpack reduction and spring drought in subalpine grasslands. Plant Biol 22:441–449. https://doi.org/10.1111/plb.13081
Bokhorst S, Bjerke JW, Melillo J, Callaghan TV, Phoenix GK (2010) Impacts of extreme winter warming events on litter decomposition in a sub-Arctic heathland. Soil Biol Biochem 42:611–617
Bokhorst S, Metcalfe DB, Wardle DA (2013) Reduction in snow depth negatively affects decomposers but impact on decomposition rates is substrate dependent. Soil Biol Biochem 62:157–164. https://doi.org/10.1016/j.soilbio.2013.03.016
Buckeridge KM, Grogan P (2008) Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl Soil Ecol 39:210–222
Buckeridge KM, Grogan P (2010) Deepened snow increases late thaw biogeochemical pulses in Mesic low arctic tundra. Biogeochemistry 101:105–121
Buckeridge KM, Banerjee S, Siciliano SD, Grogan P (2013) The seasonal pattern of soil microbial community structure in Mesic low arctic tundra. Soil Biol Biochem 65:338–347
Burghardt KT, Bradford MA, Schmitz OJ (2018) Acceleration or deceleration of litter decomposition by herbivory depends on nutrient availability through intraspecific differences in induced plant resistance traits. J Ecol 106:2380–2394. https://doi.org/10.1111/1365-2745.13002
Chapin FS, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Chapman SK, Hart SC, Cobb NS, Whitham TG, Koch GW (2003) Insect herbivory increases litter quality and decomposition: an extension of the acceleration hypothesis. Ecology 84:2867–2876
Coq S, Ibanez S (2019) Soil fauna contribution to winter decomposition in subalpine grasslands. Soil org 91:107–112. https://doi.org/10.25674/so91iss3pp107
Coq S, Nahmani J, Kazakou E, Fromin N, David JF (2020) Do litter-feeding macroarthropods disrupt cascading effects of land use on microbial decomposer activity? Basic Appl Ecol 46:24–34. https://doi.org/10.1016/j.baae.2020.03.004
Core Team R (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cornelissen JH, Van Bodegom PM, Aerts R et al (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10:619–627
Cornwell WK, Cornelissen JH, Amatangelo K et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Decker KLM, Wang D, Waite C, Scherbatskoy T (2003) Snow removal and ambient air temperature effects on forest soil temperatures in northern Vermont. Soil Sci Soc Am J 67:1234–1242
Delgado-Baquerizo M, García-Palacios P, Milla R, Gallardo A, Maestre FT (2015) Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol Biochem 81:134–142. https://doi.org/10.1016/j.soilbio.2014.11.009
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843
Gavazov KS (2010) Dynamics of alpine plant litter decomposition in a changing climate. Plant Soil 337:19–32
Gessner MO, Schwoerbel J (1989) Leaching kinetics of fresh leaf-litter with implications for the current concept of leaf-processing in streams. Arch Für Hydrobiol 115:81–90
Gosz JR, Likens GE, Bormann FH (1973) Nutrient release from decomposing leaf and branch litter in the Hubbard brook Forest, New Hampshire. Ecol Monogr 43:173–191. https://doi.org/10.2307/1942193
Grigulis K, Lavorel S, Krainer U, Legay N, Baxendale C, Dumont M, Kastl E, Arnoldi C, Bardgett RD, Poly F, Pommier T, Schloter M, Tappeiner U, Bahn M, Clément JC (2013) Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. J Ecol 101:47–57
Hobbie SE, Chapin FS (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35:327–338
Ibanez S, Bernard L, Coq S, Moretti M, Lavorel S, Gallet C (2013a) Herbivory differentially alters litter dynamics of two functionally contrasted grasses. Funct Ecol 27:1064–1074. https://doi.org/10.1111/1365-2435.12094
Ibanez S, Lavorel S, Puijalon S, Moretti M (2013b) Herbivory mediated by coupling between biomechanical traits of plants and grasshoppers. Funct Ecol 27:479–489. https://doi.org/10.1111/1365-2435.12058
Jaeger CH III, Monson RK, Fisk MC, Schmidt SK (1999) Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80:1883–1891
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Körner C (2003) Alpine plant life: functional plant ecology of High Mountain ecosystems. Springer Science & Business Media, Berlin Heidelberg
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, la Pierre K, Risch AC, Seabloom EW, Schütz M, Steenbock C, Stevens CJ, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci 112:10967–10972
Legay N, Grassein F, Robson TM, Personeni E, Bataillé MP, Lavorel S, Clément JC (2013) Comparison of inorganic nitrogen uptake dynamics following snowmelt and at peak biomass in subalpine grasslands. Biogeosci Discuss 10:8887–8917
Lemaire G, Pflimlin A (2007) Les sécheresses passées et à venir: quels impacts et quelles adaptations pour les systèmes fourragers? Fourrag 190:163–1802007
Lemaire G, Wilkins R, Hodgson J (2005) Challenges for grassland science: managing research priorities. Agric Ecosyst Environ 108:99–108
Lenth R (2018) Emmeans: estimated marginal means, aka least-squares means. R package version 1:
Lipson DA, Schmidt SK, Monson RK (1999) Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80:1623–1631
Meier CL, Bowman WD (2008) Phenolic-rich leaf carbon fractions differentially influence microbial respiration and plant growth. Oecologia 158:95–107
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Mullen RB, Schmidt SK, Jaeger CH III (1998) Nitrogen uptake during snowmelt by the snow buttercup, Ranunculus adoneus. Arct Alp Res 30:121–125
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nie Y, Wang M, Zhang W, Ni Z, Hashidoko Y, Shen W (2018) Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci Total Environ 624:407–415. https://doi.org/10.1016/j.scitotenv.2017.12.142
Nykvist N (1961) Leaching and decomposition of litter. III. Experiments on Leaf Litter of Betula verrucosa Oikos 12:249–263. https://doi.org/10.2307/3564699
Pianka ER (1970) On r-and K-selection. Am Nat 104:592–597
Piton G, Legay N, Arnoldi C, Lavorel S, Clément JC, Foulquier A (2020) Using proxies of microbial community-weighted means traits to explain the cascading effect of management intensity, soil and plant traits on ecosystem resilience in mountain grasslands. J Ecol 108:876–893. https://doi.org/10.1111/1365-2745.13327
Pitteloud C, Descombes P, Sanchez-Moreno S et al (in press) Contrasting the responses of above- and belowground herbivore communities along elevation. Oecologia
Pörtner HO, Roberts D, Masson-Delmotte V, et al (2019) IPCC special report on the ocean and cryosphere in a changing climate. IPCC Intergov Panel Clim Change Geneva Switz. https://www.ipcc.ch/srocc/
Quétier F, Thébault A, Lavorel S (2007) Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecol Monogr 77:33–52
Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer N (2010) Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 91:3463–3470
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Change Biol 18:1918–1927
Reich PB (2014) The world-wide ‘fast–slow’plant economics spectrum: a traits manifesto. J Ecol 102:275–301
Robson TM, Lavorel S, Clement J-C, Roux XL (2007) Neglect of mowing and manuring leads to slower nitrogen cycling in subalpine grasslands. Soil Biol Biochem 39:930–941. https://doi.org/10.1016/j.soilbio.2006.11.004
Robson TM, Baptist F, Clément J-C, Lavorel S (2010) Land use in subalpine grasslands affects nitrogen cycling via changes in plant community and soil microbial uptake dynamics. J Ecol 98:62–73. https://doi.org/10.1111/j.1365-2745.2009.01609.x
Saccone P, Morin S, Baptist F, Bonneville JM, Colace MP, Domine F, Faure M, Geremia R, Lochet J, Poly F, Lavorel S, Clément JC (2013) The effects of snowpack properties and plant strategies on litter decomposition during winter in subalpine meadows. Plant Soil 363:215–229
Schmidt SK, Lipson DA (2004) Microbial growth under the snow: implications for nutrient and allelochemical availability in temperate soils. Plant Soil 259:1–7
Schmidt SK, Costello EK, Nemergut DR, Cleveland CC, Reed SC, Weintraub MN, Meyer AF, Martin AM (2007) Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88:1379–1385
Schmitz OJ (2008) Effects of predator hunting mode on grassland ecosystem function. Science 319:952–954
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645–651
Silfver T, Paaso U, Rasehorn M, Rousi M, Mikola J (2015) Genotype × herbivore effect on leaf litter decomposition in Betula Pendula saplings: ecological and evolutionary consequences and the role of secondary metabolites. PLoS One 10:e0116806. https://doi.org/10.1371/journal.pone.0116806
Tao J, Zuo J, He Z, Wang Y, Liu J, Liu W, Cornelissen JHC (2019) Traits including leaf dry matter content and leaf pH dominate over forest soil pH as drivers of litter decomposition among 60 species. Funct Ecol 33:1798–1810. https://doi.org/10.1111/1365-2435.13413
Taylor BR, Bärlocher F (1996) Variable effects of air-drying on leaching losses from tree leaf litter. Hydrobiologia 325:173–182
Taylor BR, Jones HG (1990) Litter decomposition under snow cover in a balsam fir forest. Can J Bot 68:112–120
Vernay M, Lafaysse M, Hagenmuller P, et al (2019) The S2M meteorological and snow cover reanalysis in the French mountainous areas (1958 - present) [data set]
Wardle DA, Bardgett Richard D, Klironomos John N et al (2004) Ecological linkages between aboveground and belowground biota. Sci Wash DC 304:1629–1633
Wieder WR, Grandy AS, Kallenbach CM, Bonan GB (2014) Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral carbon stabilization (MIMICS) model. Biogeosciences 11:3899–3917
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Yanai Y, Toyota K, Okazaki M (2007) Response of denitrifying communities to successive soil freeze–thaw cycles. Biol Fertil Soils 44:113–119
Zeidler M, Duchoslav M, Banaš M (2014) Effect of altered snow conditions on decomposition in three subalpine plant communities. Cent Eur J Biol 9:811–822. https://doi.org/10.2478/s11535-014-0312-3
Acknowledgements
This work was funded by the ECO-SERVE project through the 2013–2014 BiodivERsA/FACCE-JPI joint call for research proposals, with the national funders ANR, NWO, FCT (BiodivERsA/001/2014), MINECO, FORMAS and SNF. This work was also funded by the Alpine Ecology Lab. We thank the municipality of Autrans-Méaudre, the local farmers and the Refuge de Gève for their authorization to access the study sites, Jonathan Crison for his hospitality, Samuel Morin for his help to retrieve meteorological data. We are grateful to Hugo Girard, Matteo Tolosano, Louise Maris and Pablo Raguet for their assistance during field work. We thank Sylvain Coq for his helpful comments on an earlier version. We are also grateful to two anonymous reviewers for their useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibanez, S., Brun, C., Millery, A. et al. Litter and soil characteristics mediate the buffering effect of snow cover on litter decomposition. Plant Soil 460, 511–525 (2021). https://doi.org/10.1007/s11104-020-04803-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04803-x