Abstract
Aims
There is a trend of increasing woody biomass in tropical savannas. Here we ask what effect this increase may have on soil carbon pools and fluxes.
Methods
Using a field experiment we determine the amount of soil carbon directly under grasses, a juvenile tree among grasses and a juvenile tree with no grasses. We also measure CO2 efflux at the soil surface and use gas wells to extract CO2 from several soil depths.
Results
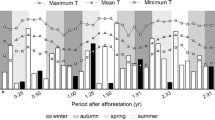
Our results show that grasses contribute substantially more than trees to both soil carbon and soil respiration. Grasses also make a disproportionate contribution to the δ13C value of SOC in the tree only treatments. The surface gas efflux data show that soil respiration increased with an increase in volumetric soil moisture and temperature and plots with both grasses and trees had higher respiration rates than plots with trees only or with grasses only.
Conclusions
The highest soil respiration is in the top 20 cm of the soil with grasses the primary contributors to both δ13CSOC and δ13CR. Any increase in woody biomass will result in a decline in SOM turnover and nitrogen mineralization rates resulting in higher SOC pools. The associated increases in SOC and above ground biomass will however be associated with negative economic and biodiversity impacts.





Similar content being viewed by others
References
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki S (eds) Mass Spectrometry of Soils. Marcel Dekker Inc, New York
Bond WJ, Midgley GF (2000) A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob Chang Biol 6:865–869
Bond WJ, van Wilgen BW (1996) Fire and Plants. Chapman and Hall, London
Bowling D, Egan J, Hall S, Risk D (2015) Environmental forcing does not induce diel or synoptic variation in the carbon isotope content of forest soil respiration. Biogeosciences 12:5143–5160
Buitenwerf R, Bond W, Stevens N, Trollope W (2012) Increased tree densities in South African savannas:> 50 years of data suggests CO2 as a driver. Glob Change Biol 18:675–684
Chen X, Hutley LB, Eamus D (2005) Soil organic carbon content at a range of north Australian tropical savannas with contrasting site histories. Plant Soil 268:161–171
Chidumayo EN (1990) Aboveground woody biomass structure and productivity in a Zambian woodland. For Ecol Manag 36:33–46
Coetsee C, Bond WJ, February EC (2010) Frequent fire affects soil nitrogen and carbon in an African savanna by changing woody cover. Oecologia 162:1027–1034. https://doi.org/10.1007/s00442-009-1490-y
Coetsee C, Gray EF, Wakeling J, Wigley BJ, Bond WJ (2013) Low gains in ecosystem carbon with woody plant encroachment in a South African savanna. J Trop Ecol 29:49–60
Craine JM, Morrow C, Stock WD (2008) Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol 179:829–836
De Castro EA, Kauffman JB (1998) Ecosystem structure in the Brazilian Cerrado: a vegetation gradient of aboveground biomass, root mass and consumption by fire. J Trop Ecol 14:263–283
Dean W, Milton S, Jeltsch F (1999) Large trees, fertile islands, and birds in arid savanna. J Arid Environ 41:61–78
Dintwe K, Okin GS (2018) Soil organic carbon in savannas decreases with anthropogenic climate change. Geoderma 309:7–16
February EC, Cook GD, Richards AE (2013) Root dynamics influence tree–grass coexistence in an Australian savanna. Austral Ecol 38:66–75. https://doi.org/10.1111/j.1442-9993.2012.02376.x
February EC, Allsopp N, Shabani T, Hattass D (2011) Coexistence of a C4 grass and a leaf succulent shrub in an arid ecosystem. The relationship between rooting depth, water and nitrogen. Plant Soil 349:253–260.
February EC, Higgins SI (2010) The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. South Afr J Bot 76:517–523. https://doi.org/10.1016/j.sajb.2010.04.001
February EC, Higgins SI (2016) Rapid leaf deployment strategies in a deciduous savanna. PLoS One 11
Fischer EM, Beyerle U, Knutti R (2013) Robust spatially aggregated projections of climate extremes. Nat Clim Chang 3:1033–1038
Frost P, Menaut JC, Walker B, Medina E, Solbrigo T (1986) Responses of savannas to stress and disturbance. International Union of Biological Sciences Special Issue 10 Paris, France
Grace J, José JS, Meir P, Miranda HS, Montes RA (2006) Productivity and carbon fluxes of tropical savannas. J Biogeogr 33:387–400
Higgins SI, Delgado-Cartay MD, February EC, Combrink HJ (2011) Is there a temporal niche separation in the leaf phenology of savanna trees and grasses? J Biogeogr 38:2165–2175. https://doi.org/10.1111/j.1365-2699.2011.02549.x
Higgins SI, Keretetse M, February EC (2015) Feedback of trees on nitrogen mineralization to restrict the advance of trees in C4 savannahs. Biol Lett 11:20150572
Holdo RM, Mack MC (2014) Functional attributes of savanna soils: contrasting effects of tree canopies and herbivores on bulk density, nutrients and moisture dynamics. J Ecol 102:1171–1182
Hudak A, Wessman C, Seastedt T (2003) Woody overstorey effects on soil carbon and nitrogen pools in South African savanna. Austral Ecol 28:173–181
Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626
Kulmatiski A, Beard KH (2013) Woody plant encroachment facilitated by increased precipitation intensity. Nat Clim Chang
Lehmann CE, Anderson TM, Sankaran M, Higgins SI, Archibald S, Hoffmann WA, Hanan NP, Williams RJ, Fensham RJ, Felfili J (2014) Savanna vegetation-fire-climate relationships differ among continents. Science 343:548–552
Makhado RA, Scholes RJ (2011) Determinants of soil respiration in a semi-arid savanna ecosystem, Kruger National Park, South Africa. Koedoe 53:00–00
Mordelet P, Menaut JC, Mariotti A (1997) Tree and grass rooting patterns in an African humid savanna. J Veg Sci 8:65–70
Mucina L, Rutherford M (eds) (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria, p 807
Nackley LL, West AG, Skowno AL, Bond WJ (2017) The nebulous ecology of native invasions. Trends Ecol Evol 32:814–824
Nel JA, Craine JM, Cramer MD (2018) Correspondence between δ13C and δ15N in soils suggests coordinated fractionation processes for soil C and N. Plant Soil 423:257–271
Pendergrass AG, Hartmann DL (2014) Changes in the distribution of rain frequency and intensity in response to global warming. J Clim 27:8372–8383
Plummer M (2003) JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003) March
Plummer M, Best N, Cowles K, Vines K (2006) CODA: convergence diagnosis and output analysis for MCMC. R News 6:7–11
R_Development_Core_Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ratter JA, Ribeiro JF, Bridgewater S (1997) The Brazilian cerrado vegetation and threats to its biodiversity. Ann Bot 80:223–230
Richards AE, Dathe J, Cook GD (2012) Fire interacts with season to influence soil respiration in tropical savannas. Soil Biol Biochem 53:90–98
Riginos C (2009) Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90:335–340. https://doi.org/10.1890/08-0462.1
Rochette P, Flanagan L, Gregorich E (1999) Separating soil respiration into plant and soil components using analyses of the natural abundance of carbon-13. Soil Sci Soc Am J 63:1207–1213
Seghieri J, Floret C, Pontanier R (1995) Plant phenology in relation to water availability: herbaceous and woody species in the savannas of northern Cameroon. J Trop Ecol 11:237–254
Venter FJ, Govender N (2012) A geomorphic and soil description of the long-term fire experiment in the Kruger National Park, South Africa. Koedoe 54:44–54
Verweij RJT, Higgins SI, Bond WJ, February EC (2011) Water sourcing by trees in a mesic savanna: Responses to severing deep and shallow roots. Environ Exp Bot 74:229–236
Ward D (2005) Do we understand the causes of bush encroachment in African savannas? Afr J Range Forage Sci 22:101–105
Wigley BJ, Augustine DJ, Coetsee C, Ratnam J, Sankaran M (2020) Grasses continue to trump trees at soil carbon sequestration following herbivore exclusion in a semi-arid African savanna. Ecology
Wigley BJ, Bond WJ, Hoffman M (2010) Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob Change Biol 16:964–976
Acknowledgements
The research was funded by the Andrew W. Mellon Foundation (#30600716). We would like to thank SANParks for permission to work in the Kruger National Park. We are grateful to Ben Wigley, Corli Coetsee, Henri Combrinck and Paola Vimercati for help with fieldwork. Thanks to Anna Richards for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
ECF and SH conceived and designed the experiments. ECF performed the experiments. ECF, SH and JP analysed the data. ECF, SH and JP wrote the manuscript.
Corresponding author
Additional information
Responsible Editor: Simon Jeffery
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
February, E., Pausch, J. & Higgins, S.I. Major contribution of grass roots to soil carbon pools and CO2 fluxes in a mesic savanna. Plant Soil 454, 207–215 (2020). https://doi.org/10.1007/s11104-020-04649-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04649-3