Abstract
Aims
Improvement cutting or harvesting can change the coverage of understory vegetation, which can significantly influence the litter decomposition process in plantations. However, difference in potential non-additive mass loss in response to understory vegetation changes is poorly studied.
Methods
A field litterbag experiment involving various litter types and treatments with no understory vegetation removal, shrub removal, herb removal and whole-understory vegetation removal was conducted to examine non-additive mass loss.
Results
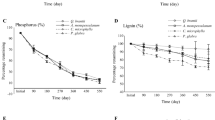
During approximately 2 years of decomposition, the decomposition rate of shrub and herb components was accelerated in the mixed litter with full understory vegetation. There was significant non-additive mass loss during decomposition in the plots with trees, shrubs and herbs, while the incidence of non-additive mass loss was lower in the plots with understory vegetation removal. Statistical analysis revealed a significant difference between the expected mass loss calculated with the data from the corresponding decomposition plots and that calculated with the data from the plots with whole-understory vegetation removal.
Conclusions
Our results show that understory vegetation removal can inhibit litter decomposition in Masson pine plantation ecosystems in subtropical China. We highlight that non-additive litter decomposition should be assessed on the basis of litter species composition and decomposition microenvironments in situ.






Similar content being viewed by others
References
Ágoston-Szabó E, Károly S, Anita K, Mária D (2017) The effects of tree species richness and composition on leaf litter decomposition in a Danube oxbow lake (Gemenc, Hungary). Fundam Appl Limnol 189:301–314
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Ball BA, Hunter MD, Kominoski JS, Swan CM, Bradford MA (2008) Consequences of non-random species loss for decomposition dynamics: experimental evidence for additive and non-additive effects. J Ecol 96:303–313
Barbe L, Mony C, Jung V, Santonja M, Bartish I, Prinzing A (2018) Functionally or phylogenetically distinct neighbours turn antagonism among decomposing litter species into synergy. J Ecol 106:1401–1414
Barrera M, Frangi JL, Ferrando JJ, Goya JF (2004) Descomposición del mantillo y liberación foliar neta de nutrientes de Austrocedrus chilensis (D. Don) Pic. Serm. Et Bizzarri en El Bolsón, Río Negro. Ecol Austral 14:99–112
Berg B, McClaugherty C (2014) Plant litter. Decomposition, humus formation, carbon sequestration, Thirdth edn. Springer, Berlin
Berg B, Berg MP, Bottner P, Box E, Breymeyer A, de Anta RC, Couteaux M, Escudero A, Gallardo A, Kratz W, Madeira M, Mälkönen E, McClaugherty C, Meentemeyer V, Muñoz F, Piussi P, Remacle J, de Santo AV (1993) Litter mass-loss rates in pine forests of Europe and eastern United States: some relationships with climate and litter quality. Biogeochemistry 20:127–153
Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA (2016) Understanding the dominant controls on litter decomposition. J Ecol 104:229–238
Chen D, Xiong H, Lin CG, He W, Zhang ZW, Wang H, Wang YJ (2019) Clonal integration benefits invasive alien plants under water variability in a native community. J Plant Ecol 12:574–582
Coûteaux MM, Hervé D, Beck S (2006) Descomposición de hojarasca y raíces en un sistema de descanso largo (Altiplano de Bolivia). Ecología en Bolivia 41:85–102
De Marco A, Meola A, Maisto G, Giordano M, De Santo AV (2011) Non-additive effects of litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 344:305–317
Forrester DI, Pares A, O’Hara C, Khanna PK, Bauhus J (2013) Soil organic carbon is increased in mixed-species plantations of eucalyptus and nitrogen-fixing acacia. Ecosystems 16:123–132
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Harrison AF (1971) The inhibitory effect of oak leaf litter tannins on the growth of fungi, in relation to litter decomposition. Soil Biol Biochem 3:167–172
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol S 36:191–218
He W, Wu FZ, Zhang DJ, Yang WQ, Bo T, Zhao YY, Wu QQ (2015) The effects of forest gaps on cellulose degradation in the foliar litter of two shrub species in an alpine fir forest. Plant Soil 393:109–122
He W, Wu FZ, Yang WQ, Zhang DJ, Xu ZF, Tan B, Zhao YY, Justine MF (2016) Gap locations influence the release of carbon, nitrogen and phosphorus in two shrub foliar litter in an alpine fir forest. Sci Rep UK 6:22014
He W, Ma ZY, Pei J, Teng MJ, Zeng LX, Yan ZG, Huang ZL, Zhou ZX, Wang PC, Luo X, Xiao WF (2019) Effects of predominant tree species mixing on lignin and cellulose degradation during leaf litter decomposition in the three gorges reservoir, China. Forests 10:360
Keith AM, Van Der Wal R, Brooker RW, Osler GHR, Chapman SJ, Burslem DFRP, Elston DA (2008) Increasing litter species richness reduces variability in a terrestrial decomposer system. Ecology 89:2657–2664
Lecerf A, Risnoveanu G, Popescu C, Gessner MO, Chauvet E (2007) Decomposition of diverse litter mixtures in streams. Ecology 88:219–227
Lu R (1999) Soil and agro-chemical analytical methods. China. Agricultural Science and Technology Press, Beijing, pp 146–195 (in Chinese, English abstract)
Lu WJ, Liu N, Zhang YJ, Zhou JQ, Guo YR, Yang X (2017) Impact of vegetation community on litter decomposition: evidence from a reciprocal transplant study with 13C labeled plant litter. Soil Biol Biochem 112:248–257
Makkonen K, Berg MP, van Logtestijn RSP, van Hal JR, Aerts R (2013) Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 122:987–997
Mao B, Zeng DH (2012) Non-additive effects vary with the number of component residues and their mixing proportions during resdue mixture decomposition: a microcosm study. Geoderma 170:112–117
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Steinwandter M, Schlick-Steiner BC, Steiner FM, Seeber J (2019) One plus one is greater than two: mixing litter types accelerates decomposition of low-quality alpine dwarf shrub litter. Plant Soil 438:405–419
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press
Taylor BR, Parkinson D, Parsons WF (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Thiessen S, Gleixner G, Wutzler T, Reichstein M (2013) Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass – an incubation study. Soil Biol Biochem 57:739–748
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Veen GF, Freschet GT, Ordonez A, Wardle DA (2015) Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124:187–195
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Wall DH, Bradford MA, John MST, Trofymow JA, Pelletier VB, Bignell DE, Zou XM (2008) Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Chang Biol 14:2661–2677
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Wu DD, Li TT, Wan SQ (2013) Time and litter species composition affect litter-mixing effects on decomposition rates. Plant Soil 371:355–366
Wu FZ, Peng CH, Yang WQ, Zhang J, Han Y, Mao T (2014) Admixture of alder (Alnus formosana) litter can improve the decomposition of eucalyptus (Eucalyptus grandis) litter. Soil Biol Biochem 73:115–121
Yue K, Yang WQ, Tan B, Peng Y, Huang CP, Xu ZF, Ni XY, Yang Y, Zhou W, Zhang L, Wu FZ (2019) Immobilization of heavy metals during aquatic and terrestrial litter decomposition in an alpine forest. Chemosphere 216:419–427
Zeng LX, He W, Teng MJ, Luo X, Yan ZG, Huang ZL, Zhou ZX, Wang PC, Xiao WF (2018) Effects of mixed leaf litter from predominant afforestation tree species on decomposition rates in the three gorges reservoir, China. Sci Total Environ 639:679–686
Acknowledgments
We are grateful to Mr. Hongdong Pang and Pro. Hongxia Cui of Hubei’s Academy of Forestry and Linshan Sun of the Hubei Taizishan Forest Farm Administration Bureau for assistance with the understory vegetation survey. This work was supported by the National Key R&D Program of China (No. 2016YFD0600201), the National Natural Science Foundation of China (Nos. 31800518, 31870611 and 31400531), the Fundamental Research Funds for the Central Universities (Nos. 2662018PY084 and 2662018QD059), Hubei Province Key Technology Innovation Project (2016ABA111) and the Hubei Provincial Natural Science Foundation of China (2018CFB374).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Luca Bragazza .
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 254 KB)
Rights and permissions
About this article
Cite this article
He, W., Xu, X., Zhang, C. et al. Understory vegetation removal reduces the incidence of non-additive mass loss during leaf litter decomposition in a subtropical Pinus massoniana plantation. Plant Soil 446, 529–541 (2020). https://doi.org/10.1007/s11104-019-04378-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04378-2