Abstract
Aims
To screen plant-associated Burkholderia strains for plant probiotic traits including allelochemical metabolism and understand their role on rice allelopathy using a bacteriostatic-dose of antibiotic.
Methods
Burkholderia sp. LS-044, CC-Al74 and CC-3XP9 were screened for plant probiotic traits. Rice (Oryza sativa ssp. japonica cv. Tainung 71) root endophytic isolate LS-044 was subjected to multilocus sequence typing, antibiotic assay, and seed germination bioassay under ferulic acid (FA; 0–200 ppm) and bacteriostatic-dose of meropenem (4 ppm). FA metabolism and cell viability were assessed through alamarBlue (AB) assay.
Results
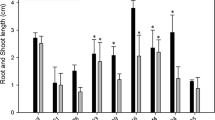
All tested Burkholderia strains exhibited multiple plant probiotic traits. LS-044, a meropenem-sensitive novel member of Burkholderia cepacia complex displayed rapid metabolism of multiple allelochemicals. Bacteriostatic-dose of meropenem improved germination index (GI) of inoculated seed under FA treatments. Meropenem promoted GI by controlling bacterial FA metabolism and prolonging the cell viability, as evident through AB assay. Uncontrolled bacterial growth under high-dose FA found to be phytotoxic to rice than FA alone.
Conclusions
Burkholderia sp. LS-044 is a potential allelochemical-metabolizing bacterium, which possibly plays a key role in rice allelopathy. Bacteriostatic-dose of meropenem mitigates autotoxicity by controlling and prolonging allelochemical metabolism of rice-associated LS-044.




Similar content being viewed by others
Abbreviations
- AB:
-
alamarBlue
- Bcc:
-
Burkholderia cepacia complex
- DMRT:
-
Duncan’s multiple range test
- FA:
-
ferulic acid
- FA0:
-
0 ppm FA
- FA50:
-
50 ppm FA
- FA200:
-
200 ppm FA
- FA0 + Mer:
-
0 ppm FA +4 ppm meropenem
- FA50 + Mer:
-
50 ppm FA +4 ppm meropenem
- FA200 + Mer:
-
200 ppm FA +4 ppm meropenem
- GI:
-
germination index
- IAA:
-
Indole-3-acetic acid
- Mer:
-
meropenem
- +Mer:
-
bacteriostatic-dose of meropenem
- MICMer :
-
minimum inhibitory concentration of meropenem
- NA:
-
nutrient agar
- NB:
-
nutrient broth
- OD:
-
optical density
- PDA:
-
potato dextrose agar
- pltC :
-
gene encoding pyoluteorin
- ppm:
-
parts per million
- prnD :
-
gene encoding pyrrolnitrin
- RH ratio:
-
radicle:hypocotyl ratio
- TN71:
-
Tainung 71 without LS-044 inoculation
- TN71*:
-
Tainung 71 with LS-044 inoculation
- VOC:
-
volatile organic compounds
References
Aaron SD, Ferris W, Henry DA, Speert DP, MacDonald NE (2000) Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am J Respir Crit Care Med 161:1206–1212
Arima K, Imanaka H, Kousaka M, Fukuda A, Tamura G (1965) Studies on pyrrolnitrin a new antibiotic .I. Isolation and properties of pyrrolnitrin. J Antibiot 18:201–204
Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME (2009) Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Agents 33:394–404
Baldwin A, Mahenthiralingam E, Thickett KM, Honeybourne D, Maiden MCJ, Govan JR, Speert DP, LiPuma JJ, Vandamme P, Dowson CG (2005) Multilocus sequence typing scheme that provides both species and strain differentiation for the Burkholderia cepacia complex. J Clin Microbiol 43:4665–4673
Bevivino A, Sarrocco S, Dalmastri C, Tabacchioni S, Cantale C, Chiarini L (1998) Characterization of a free-living maize-rhizosphere population of Burkholderia cepacia: effect of seed treatment on disease suppression and growth promotion of maize. FEMS Microbiol Ecol 27:225–237
Bi HH, Zeng RS, Su LM, An M, Luo SM (2007) Rice allelopathy induced by methyl jasmonate and methyl salicylate. J Chem Ecol 33:1089–1103
Blum U (2004) Fate of phenolic allelochemicals in soils – the role of soil and rhizosphere microorganisms. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Allelopathy: chemistry and mode of action of allelochemicals. CRC Press, Boca Raton, pp 57–76
Chaudhary HJ, Peng G, Hu M, He Y, Yang L, Luo Y, Tan Z (2012) Genetic diversity of endophytic diazotrophs of the wild rice, Oryza alta and identification of the new diazotroph, Acinetobacter oryzae sp. nov. Microb Ecol 63:813–821
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Chi WC, Chen YA, Hsiung YC, Fu SF, Chou CH, Trinh NN, Chen YC, Huang HJ (2013) Autotoxicity mechanism of Oryza sativa: transcriptome response in rice roots exposed to ferulic acid. BMC Genomics 14:351
Chou CH (1986) The role of allelopathy in subtropical agroecosystems in Taiwan. In: Putnam AR, Tang CS (eds) The science of allelopathy. John Wiley and Sons Inc., New York, USA, pp 57–73
Chou CH (1990) The role of allelopathy in agroecosystems: studies from tropical Taiwan. In: Gliessman SR (ed) Agroecology: researching the ecological basis for sustainable agriculture. Ecological studies no. 78. Springer-Verlag, Berlin, pp 105–121
Chou CH, Chiou SJ (1979) Autointoxication mechanism of Oryza sativa. II. Effects of culture treatments on the chemical nature of paddy soil and on rice productivity. J Chem Ecol 5:839–859
Chou CH, Lin HJ (1976) Autointoxication mechanism of Oryza sativa. I. Phytotoxic effcets of decomposing rice residues in soil. J Chem Ecol 2:353–367
Chou CH, Chiang YC, Chang HH (1981) Autointoxication mechanism of Oryza sativa. III. Effect of temperature on phytotoxin production during rice straw decomposition in soil. J Chem Ecol 7:741–752
Cruze JA, Singer JT, Finnerty WR (1979) Conditions for quantitative transformation in Acinetobacter calcoaceticus. Curr Microbiol 3:129–132
Dalmastri C, Baldwin A, Tabacchioni S, Bevivino A, Mahenthiralingam E, Chiarini L, Dowson C (2007) Investigating Burkholderia cepacia complex populations recovered from Italian maize rhizosphere by multilocus sequence typing. Environ Microbiol 9:1632–1639
Dalton BR (1999) The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: methodological limitation in establishing conclusive proof of allelopathy. In: Inderjit DKMM, Foy CJ (eds) Principles and practices in plant ecology: Allelochemical interactions. CRC Press, Boca Raton, Florida, pp 57–74
de Souza JT, Raaijmakers JM (2003) Polymorphisms within the prnD and pltC genes from pyrrolnitrin and pyoluteorin-producing Pseudomonas and Burkholderia spp. FEMS Microbiol Ecol 43:21–34
Di Cello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C (1997) Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol 63:4485–4493
Dilday RH, Yan WG, Moldenhauer KAK, Gravois KA (1998) Allelopathic activity in rice for controlling major aquatic weeds. In: Olofsdotter M (ed) Allelopathy in Rice. Los Banos, IRRI, Philippines, pp 7–26
Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes - characterization of a gene coding for 16S-ribosomal RNA. Nucleic Acids Res 17:7843–7853
El-Banna N, Winkelmann G (1998) Pyrrolnitrin from Burkholderia cepacia: antibiotic activity against fungi and novel activities against streptomycetes. J Appl Microbiol 85:69–78
Hameed A, Yeh MW, Hsieh YT, Chung WC, Lo CT, Young LS (2015) Diversity and functional characterization of bacterial endophytes dwelling in various rice (Oryza sativa L.) tissues, and their seed-borne dissemination into rhizosphere under gnotobiotic P-stress. Plant Soil 394:177–197
Hammer PE, Burd W, Hill DS, Ligon JM, van Pée KH (1999) Conservation of the pyrrolnitrin biosynthetic gene cluster among six pyrrolnitrin-producing strains. FEMS Microbiol Lett 180:39–44
Hardoim PR, Hardoim CCP, van Overbeek LS, van Elsas JD (2012) Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS One 7:e30438
He H, Chen X, Lin R, Lin W, He H, Jia X, Xiong J, Shen L, Liang Y (2005a) Chemical components of root exudates from allelopathic rice accession PI312777 seedlings. Chin J Appl Ecol 16:2383–2388
He H, He H, Lin W, Chen X, Jia X, Xiong J, Shen L, Liang Y (2005b) Terpenoids in root exudates of different allelopathic rice varieties. Chin J Appl Ecol 16:732–736
Heiner CR, Hunkapiller KL, Chen SM, Glass JI, Chen EY (1998) Sequencing multimegabase-template DNA with BigDye terminator chemistry. Genome Res 8:557–561
Hernández-Rodríguez A, Heydrich-Pérez M, Diallo B, El Jaziri M, Vandeputte OM (2010) Cell-free culture medium of Burkholderia cepacia improves seed germination and seedling growth in maize (Zea mays) and rice (Oryza sativa). Plant Growth Regul 60:191–197
Hikichi Y, Egami H, Oguri Y, Okuno T (1998) Fitness for survival of Burkholderia glumae resistant to oxolinic acid in rice plant. Ann Phytopathol Soc Jpn 64:147–152
Inderjit (1996) Plant phenolics in allelopathy. Bot Rev 62:186–202
Inglis TJJ, Rodrigues F, Rigby P, Norton R, Currie BJ (2004) Comparison of the susceptibilities of Burkholderia pseudomallei to meropenem and ceftazidime by conventional and intracellular methods. Antimicrob Agents Chemother 48:2999–3005
Inglis TJJ, Hahne DR, Merritt AJ, Clarke MW (2015) Volatile-sulfur-compound profile distinguishes Burkholderia pseudomallei from Burkholderia thailandensis. J Clin Microbiol 53:1009–1011
Kai M, Effmert U, Berg G, Piechulla B (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol 187:351–360
Kang SM, Khan AL, Hussain J, Ali L, Kamran M, Waqas M, Lee IJ (2012) Rhizonin A from Burkholderia sp. KCTC11096 and its growth promoting role in lettuce seed germination. Molecules 17:7980–7988
Kato-Noguchi H (2004) Allelopathic substance in rice root exudates: rediscovery of momilactone B as an allelochemical. J Plant Physiol 161:271–276
Kim KU, Shin DH (2008) Progress and prospect of rice allelopathy research. In: Zeng RS, Mallik AU, Luo SM (eds) Allelopathy in sustainable agriculture and forestry. Springer, New York, pp 189–213
Lin WX, Kim KU, Shin DH (2000) Rice allelopathic potential and its modes of action on barnyardgrass (Echinochloa crusgalli L.). Allelopathy J 7:215–224
Lin TF, Huang HI, Shen FT, Young CC (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-A174. Bioresour Technol 97:957–960
Liu HW, Carvalhais LC, Crawford M, Singh E, Dennis PG, Pieterse CMJ, Schenk PM (2017) Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.02552
Lou MM, Zhang LX, Su T, Xie GL (2007) Genomovars of Burkholderia cepacia complex from rice rhizosphere and clinic in China. Rice Sci 14:229–234
Lu G, Moriyama EN (2004) Vector NTI, a balanced all-in-one sequence analysis suite. Brief Bioinform 5:378–388
Mano H, Morisaki H (2008) Endophytic bacteria in the rice plant. Microbes Environ 23:109–117
Nowak-Thompson B, Chaney N, Wing JS, Gould SJ, Loper JE (1999) Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5. J Bacteriol 181:2166–2174
Olofsdotter M, Rebulanan M, Madrid A, Dali W, Navarez D, Olk DC (2002) Why phenolic acids are unlikely allelochemicals in rice. J Chem Ecol 28:229–242
Partida-Martinez LP, de Looß CF, Ishida K, Ishida M, Roth M, Buder K, Hertweck C (2007) Rhizonin, the first mycotoxin isolated from the zygomycota, is not a fungal metabolite but is produced by bacterial endosymbionts. Appl Environ Microbiol 73:793–797
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Peeters E, Nelis HJ, Coenye T (2009) In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria. J Antimicrob Chemother 64:801–809
Rhodes KA, Schweizer HP (2016) Antibiotic resistance in Burkholderia species. Drug Resist Updat 28:82–90
Rice EL (1984) Allelopathy, 2nd edn. Academic Press, New York
Seal AN, Pratley JE, Haig T, An M (2004) Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J Chem Ecol 30:1647–1662
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M, Hurek T, Sarkar A, Bodrossy L, van Overbeek L, Brar D, van Elsas JD, Reinhold-Hurek B (2012) Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant-Microbe Interact 25:28–36
Singh HP, Batish DR, Kohli RK (1999) Autotoxicity: concept, organisms, and ecological significance. CRC Crit Rev Plant Sci 18:757–772
Sun L, Qiu F, Zhang X, Dai X, Dong X, Song W (2008) Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microb Ecol 55:415–424
Sundin GW, Wang N (2018) Antibiotic resistance in plant-pathogenic bacteria. Annu Rev Phytopathol 56:161–180
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications Ltd., Oxford, UK, ISBN: 0632064102
Watts D, MacBeath JR (2001) Automated fluorescent DNA sequencing on the ABI PRISM 310 genetic analyzer. Methods Mol Biol 167:153–170
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols a guide to methods and applications. Academic Press, New York, pp 315–322
Whitehead DC (1964) Identification of p-hydroxybenzoic, vanillic, p-coumaric and ferulic acids in soils. Nature 202:417–418
Xie XG, Dai CC (2015) Degradation of a model pollutant ferulic acid by the endophytic fungus Phomopsis liquidambari. Bioresour Technol 179:35–42
Yang CM, Lee CN, Chou CH (2002) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: I. Inhibition of supply-orientation. Bot Bull Acad Sinica 43:299–304
Yang CM, Chang IF, Lin SJ, Chou CH (2004) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: II. Stimulation of consumption-orientation. Bot Bull Acad Sinica 45:119–125
Yoshii A, Moriyama H, Fukuhara T (2012) The novel kasugamycin 2'-N-acetyltransferase gene aac(2′)-IIa, carried by the IncP island, confers kasugamycin resistance to rice-pathogenic bacteria. Appl Environ Microbiol 78:5555–5564
Young LS, Hameed A, Peng SY, Shan YH, Wu SP (2013) Endophytic establishment of the soil isolate Burkholderia sp. CC-Al74 enhances growth and P-utilization rate in maize (Zea mays L.). Appl Soil Ecol 66:40–47
Zamzuri NA, Abd-Aziz S, Rahim RA, Phang LY, Alitheen NB, Maeda T (2014) A rapid colorimetric screening method for vanillic acid and vanillin-producing bacterial strains. J Appl Microbiol 116:903–910
Zeng RS, Luo SM, Shi YH, Shi MB, Tu CY (2001) Physiological and biochemical mechanism of allelopathy of secalonic acid F on higher plants. Agron J 93:72‒79
Zhao K, Penttinen P, Zhang X, Ao X, Liu M, Yu X, Chen Q (2014) Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol Res 169:76–82
Zhou JY, Chen YH, Tabibi S, Alba L, Garber E, Saiman L (2007) Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 51:1085–1088
Acknowledgements
Authors would like to thank anonymous reviewers for their critical comments on this manuscript and Ms. Yu-Ting Hseih for her technical assistance. This work was financially supported by the Ministry of Science and Technology (Taiwan) under Grant No. MOST 107-2634-F-005-002 and by the “Innovation and Development Center of Sustainable Agriculture” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Birgit Mitter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 579 kb)
Rights and permissions
About this article
Cite this article
Hameed, A., Shahina, M., Young, LS. et al. Bacteriostatic stimulus of meropenem on allelochemical-metabolizing Burkholderia sp. LS-044 mitigates ferulic acid autotoxicity in rice (Oryza sativa ssp. japonica cv. Tainung 71). Plant Soil 443, 73–86 (2019). https://doi.org/10.1007/s11104-019-04195-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04195-7