Abstract
Background and aims
Evidence from growth chamber and greenhouse experiments indicates that the rhizosphere priming effect (RPE, stimulation or suppression of soil organic matter (SOM) decomposition by live roots and their rhizospheric biota) may be sensitive to environmental factors such as temperature and level of sunlight. However, little is known about potential variations of the RPE under different latitudinal regions that vary in temperature and sunlight levels.
Methods
We explored the RPE of soybean (Glycine max) and cottonwood (Populus × euramericana cv. ‘74/76’) at two different latitudinal locations under variable conditions of light and temperature for two growing seasons using a natural 13C tracer method.
Results
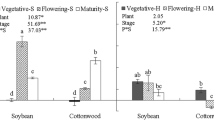
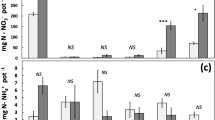
Different plant species at different time of season produced significantly different RPEs. The RPE of both soybean and cottonwood was small at the initial stage when the plants were small. The RPE of soybean ranged from 0.4% to135%, reached the highest level at the flowering stage and declined afterward. The RPE of cottonwood ranged from −17% to 121% and tended to increase in both growing seasons. The cumulative RPE of soybean was significantly higher than that of cottonwood at both locations and during the two growing seasons. Overall the cumulative RPE values were larger at low latitude than at high latitude. The cumulative RPE of soybean at the low latitudinal location was 1.7 times higher than at the high latitudinal location. The RPE of both plant species was significantly related to specific rhizosphere respiration, but negatively related to soil dissolved total nitrogen content.
Conclusions
The RPE of both plant species varied at both locations during the two growing seasons. Particularly for soybean, the cumulative RPE was higher at the low latitudinal location than at the high latitudinal location, possibly because of higher light levels. Our results further indicate that plant species, time of season, specific rhizosphere respiration, and soil dissolved total nitrogen are important variables in controlling the RPE. Overall, these results provide the needed support for using RPE data in a broader context.





Similar content being viewed by others
Abbreviations
- RPE:
-
Rhizosphere priming effect
- SOM:
-
Soil organic matter
- PAR:
-
Photosynthetically active radiation
- PVC:
-
Polyvinyl chloride
- DAP:
-
Days after planted
- CRDS:
-
Cavity ring-down spectroscopy
- MBC:
-
Microbial biomass carbon
- DOC:
-
Dissolved organic carbon
- DTN:
-
Dissolved total nitrogen
- SRR:
-
Specific rhizosphere respiration
References
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Cheng W, Johnson DW, Fu S (2003) Rhizosphere effects on decomposition: controls of plant species, phenology, and fertilization. Soil Sci Soc Am J 67:1418–1427
Cheng W, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44
Chowdhury S, Farrell M, Bolan N (2014) Priming of soil organic carbon by malic acid addition is differentially affected by nutrient availability. Soil Biol Biochem 77:158–169
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Dijkstra FA, Cheng W (2007) Moisture modulates rhizosphere effects on C decomposition in two different soil types. Soil Biol Biochem 39:2264–2274
Dijkstra FA, Carrillo Y, Pendall E, Morgan J (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:216
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC (2013) Stoichiometry constrains microbial response to root exudation- insights from a model and a field experiment in a temperate forest. Biogeosciences 10:821–838
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96
Fu S, Cheng W (2002) Rhizosphere priming effects on the decomposition of soil organic matter in C4 and C3 grassland soils. Plant Soil 238:289–294
Fu S, Cheng W, Susfalk R (2002) Rhizosphere respiration varies with plant species and phenology: a greenhouse pot experiment. Plant Soil 239:133–140
Gregory PJ, Atwell BJ (1991) The fate of carbon in pulse-labelled crops of barley and wheat. Plant Soil 136:205–213
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–292
Hopkins F, Gonzalez-Meler MA, Flower CE, Lynch DJ, Czimczik C, Tang J, Subke JA (2013) Ecosystem-level controls on root-rhizosphere respiration. New Phytol 199:339–351
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analysis. Soil Biol Biochem 111:78–84
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33:1915–1925
Kuzyakov Y, Cheng W (2004) Photosynthesis controls of CO2 efflux from maize rhizosphere. Plant Soil 263:85–99
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. J Plant Nutr Soil Sci 163:421–431
Palta JA, Gregory PJ (1997) Drought affects the fluxes of carbon to roots and soil in 13C pulse-labelled plants of wheat. Soil Biol Biochem 29:1395–1403
Phillips RP, Fahey TJ (2006) Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 87:1302–1313
Schimel DS (1995) Terrestrial ecosystems and the carbon-cycle. Glob Chang Biol 1:77–91
Wang X, Tang C, Severi J, Butterly CR, Baldock JA (2016) Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol 211:863–873
Zhu B, Cheng W (2011a) Rhizosphere priming effect increases the temperature sensitivity of soil organic matter decomposition. Glob Chang Biol 17:2172–2183
Zhu B, Cheng W (2011b) 13C isotope fractionation during rhizosphere respiration of C3 and C4 plants. Plant Soil 342:277–287
Zhu B, Cheng W (2012) Nodulated soybean enhances rhizosphere priming effects on soil organic matter decomposition more than non-nodulated soybean. Soil Biol Biochem 51:56–65
Acknowledgements
We thank National Field Research Station of Shenyang Agroecosystems for providing climate data. We also thank Mr. Cui of Heihe Branch, Heilongjiang Academy of Agricultural Sciences for experimental setup and sampling.
Funding
This work was supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB15030302), the National Natural Science Foundation of China under the Grant numbers of 31,470,527 and 31,470,625, and the U.S. National Science Foundation (Grant No. DEB-1354098).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Sven Marhan.
Electronic supplementary material
ESM 1
(DOCX 51 kb)
Rights and permissions
About this article
Cite this article
Su, T., Dijkstra, F.A., Wang, P. et al. Rhizosphere priming effects of soybean and cottonwood: do they vary with latitude?. Plant Soil 420, 349–360 (2017). https://doi.org/10.1007/s11104-017-3396-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3396-5