Abstract
Background and aims
Understanding the potential effects of iron toxicity on plant development is important when constructing new wetland from iron-rich sediment. We aim to study plant species-specific effects of iron toxicity when grown in the iron-rich sediments of lake Markermeer (the Netherlands).
Methods
Using three sediment sources that varied in total Fe and Fe-P concentrations, we performed a greenhouse experiment to study the development of three wetland species that differ in their tolerance to iron and utilization capacity of Fe-P: Rumex maritimus, Phragmites australis and Eupatorium cannabinum.
Results
Phragmites australis was the only species that developed an epidermis-damaging iron plaque on its roots. Plaque formation mainly depended on the Fe(III) and Fe-P concentration of the sediment, which led to different nutrient imbalances in leaves. All three species showed reduced growth compared to the control substrate, which could not be linked to indirect Fe toxicity. In contrast, direct Fe toxicity following the uptake of Fe could not be excluded as a mechanism potentially explaining our results, and this result warrants further examination in longer-term experiments.
Conclusions
Our results highlight the importance of considering the Fe and Fe-P availability in sediments, as these properties may constrain plant performance and delay the development of pioneer ecosystems in wetland construction sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of ecological engineering is implemented globally nowadays and aims to use environmental technology that is tuned to ecosystem services (Mitsch 1998; Odum and Odum 2003; Temmerman et al. 2013). Often, plants are used as ecological engineers as they directly interact with the physical, chemical and biological components in the soil (Ehrenfeld et al. 2005), thereby potentially facilitating ecosystem development. Especially when constructing wetlands, the ability of plants to modify the biogeochemical conditions by radial oxygen loss (ROL) is important as it enables them to cope with the high concentration of phytotoxins typically found in these waterlogged soils (e.g. Fe2+, Mn2+, S−) (Blom 1999; Lamers et al. 2012). In constructed wetlands build from iron-rich sediments, this ability could be an important characteristic as optimal growing conditions are required for plants to fully operate as ecological engineers.
Several studies showed that the iron availability in wetland soils is an important factor influencing plant species distribution, owing to the different strategies of plants to cope with excessive amounts of iron (Snowden and Wheeler 1993, 1995; Van der Welle et al. 2007; Geurts et al. 2009). High iron availability in soils can lead to direct iron toxicity or indirect iron toxicity in plants. Direct iron toxicity occurs when an excessive uptake of Fe damages cell structures, leading to reduced plant growth and injury to foliage (Wheeler et al. 1985; Laan et al. 1991; Ayeni et al. 2014) whereas indirect iron toxicity occurs when iron precipitates on roots, forming an iron plaque that acts as a barrier against iron (Snowden and Wheeler 1995; Tripathi et al. 2014). Although indirect iron toxicity is an exclusion mechanism that prevents the excessive uptake of iron – i.e. direct iron toxicity – it also inhibits nutrient uptake by damaging the epidermis surface of roots (Jørgenson et al. 2013). For example, Snowden and Wheeler (1995) found that increased iron concentrations in the soil induced a stronger decrease in shoot N concentrations than in root N concentrations, whereas the opposite was found for potassium (K). Hence, uptake and translocation mechanisms are affected in different ways depending on the type of nutrient.
The type of iron plaque that precipitates on plant roots also determines the plant response to indirect iron toxicity, especially with respect to phosphorus (P) uptake. Iron can precipitate with P, forming a yellow-grey colored plaque, or without P, forming an ochreous colored plaque (Snowden and Wheeler 1995). Plants that favor co-precipitation of P induce a higher uptake of P but severely reduce translocation of P, leading to more phosphorus stress in the shoot (Snowden and Wheeler 1995; Xu et al. 2009). However, little is known about the relationship between the composition of iron in sediments and the type of iron plaque formed on the roots.
Studying how plants respond to direct and indirect iron toxicity is important if iron-rich sediments are used as a foundation for newly constructed wetlands. Sufficient plant growth is a prerequisite for vegetation to act as ecological engineers and speed up the development of these ecosystems. Wetland construction in lake Markermeer, an artificial lake in the Netherlands located northeast of Amsterdam, is an example. See Fig. 1a for an artistic impression of these wetlands.
Nowadays, a soft, clay-rich layer is causing serious turbidity problems in lake Markermeer. The soft clay-rich layer is produced by bioturbation and physical weathering and continuously resuspends as a result of wave action (Van Kessel et al. 2008; Vijverberg et al. 2011; De Lucas Pardo et al. 2013). From an ecological point of view, removing the soft clay-rich layer is necessary as it is causing serious turbidity problems (Fig. 1b) with severe consequences for the lake’s biodiversity (Noordhuis et al. 2014). To improve the ecological conditions, it is planned to dredge a part of the soft clay-rich lake-bed sediment and use this as a building material in constructing approximately 10,000 ha of wetland. In an earlier study, Saaltink et al. (2016) found that the soft, clay-rich layer contains significant amounts of pyrite (FeS2) and iron-bound phosphorus and they hypothesized that the main bottleneck preventing prompt development of ecosystems within the newly constructed wetland sites could be a form of iron toxicity. High belowground production of pioneering vegetation is especially important when using vegetation as ecological engineers for building these wetlands from soft mud, as roots have stabilizing capabilities, reduce erosion, and will increase the consolidation process.
The aim of this study is to identify the effects of iron toxicity in plants when grown in the iron-rich sediments of lake Markermeer. Consequently, we formulated two subsidiary objectives to reach this aim: 1) determine the presence and composition of iron plaque on the plant roots and show that this plaque formation depends on the amount of iron and iron-bound phosphorus present in the sediments; 2) monitor concentrations of Fe, N, P, and K in leaves and roots and link changes in plant nutrient stoichiometry – such as changes in tissue N:P ratio due to altered uptake and translocation of N and/or P – to the presence and composition of iron plaque. We use three wetland species that commonly occur in the Markermeer lake area and that differ in their tolerance to iron. Moreover, we use three sediments from different locations in lake Markermeer that highly vary in their Fe, Fe-P and nutrient concentrations. We hypothesize that plant species with high radial oxygen loss will produce substantial amounts of iron plaque, whereas plant species with low radial oxygen loss will produce little or no iron plaque. We expect that more P is co-precipitated with Fe on roots in sediments that contain high amounts of Fe-P. Moreover, the presence of iron plaque as well as the type of iron-plaque is expected to induce changes in plant nutrient stoichiometry in plants. This study will enhance knowledge on plant-soil interactions and specifically how excessive iron influences vegetation performance in terms of plant growth, plaque formation and nutrient stoichiometry.
Materials and methods
Experimental design
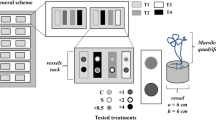
A greenhouse experiment was conducted at the greenhouse test facility of Utrecht University in the period July – September 2015. We selected three plant species that differ in their ability to cope with iron toxicity: Rumex maritimus (golden dock), Phragmites australis (common reed) and Eupatorium cannabinum (hemp-agrimony). All three species are commonly found in the Netherlands and usually grow on wet soils. Rumex maritimus is an annual, dicotyledonous species which can cope sufficiently with high iron concentrations in terms of growth despite observations of substantial leaf injury (Laan et al. 1991); P. australis is a perennial, monocotyledonous species that is moderately tolerant to Fe, showing marked signs of iron toxicity at high Fe concentrations (Snowden and Wheeler 1995); and E. cannabinum is a perennial, dicotyledonous species with low tolerance to high Fe concentrations, showing severe signs of iron toxicity (Snowden and Wheeler 1995). Plants were grown from seeds for c. 40 days on nutrient-poor turf soil before transplantation into the experimental sediments.
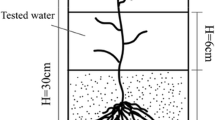
Lake bed sediment was collected by mechanical dredging at two points in Markermeer: the southern part (coordinates 52.3795 N; 5.0209E, sampled in June 2014) and the northern part (coordinates 52.5462 N; 5.3878E, sampled in March 2015). Fast changes in the geochemical composition due to seasonal effects are not expected as the buffer capacities of these sediments are large (Saaltink et al. 2016). From these sampling points, three sediment types were used: (1) the southern, well consolidated, Zuiderzee deposit of Holocene origin (10–50 cm depth), (2) the soft clay-rich layer which is found on top of this deposit (0–10 cm depth), and (3) also a soft clay-rich layer at the northern part (0–10 cm depth). Sediments were stored in air-tight containers at 4 °C prior to the start of the experiment. As a fourth sediment type, potting soil was used as a control treatment. As plant growth impairment by iron toxicity was not likely in the control treatment, it provided the reference needed to assess growth impairment in the Markermeer sediments. Pots (diameter 5 cm, depth 18 cm) with a perforated base were filled with one of the four sediment types. A batch of 30 pots were placed in small basins and filled with water to a level of 6 cm (12 cm below soil surface). The water was treated by reverse osmosis, after which NaCl was dissolved to create a solution of 30 10−6 mol L−1. This value reflects the average NaCl concentration in rainwater in the Netherlands for the period 2012–2013 (LMRe 2014). One sediment type per basin was used to prevent any crossing-over between sediment conditions.
A seedling was planted in each pot after it was filled with sediment. For each sediment type – species combination, 15 pots were made to account for the number of harvests (3) and replicates (5). Seedlings from other plants that spontaneously emerged in the pots were removed immediately.
Data collection
Roots, stems and leaves were harvested, separated, air-dried and weighed prior to the start of the experiment and after 28, 56, and 84 days. In addition, flowers were harvested from R. maritimus and E. cannabinum after 56 and 84 days in the control sediment and in the sediment with an intermediate concentration of iron and after 84 days in the sediments with high and low concentrations of iron. Phragmites australis did not flower in any of the sediment treatments during the experiment. The air-dried material was then ground and analyzed using total reflection X-ray fluorescence (S2 Picofox, Bruker) to determine tissue contents of Fe, K, and P. Nitrogen was determined on a CN elemental analyzer (NA1500, Fisons Instruments). At the start of the experiment, the biomass of a single seedling was too low to enable chemical analysis. Therefore, seedlings were harvested and processed in sets of 10 per replicate. Part of a lateral root of the harvest after 84 days was randomly selected for micro X-ray fluorescence spectrometry (Orbis PC, Edax) to determine the elemental composition of the root surface.
Before the start of the experiment, soil samples were freeze-dried and stored in anoxic conditions prior to geochemical analysis in order to preserve the solid P and Fe speciation in the soil. A series of geochemical analyses was performed on the field samples of the sediments. The total concentrations of Fe, P, and K were determined using ICP-OES (Arcos, Spectro) following aqua regia destruction. Soil N was measured on a CN elemental analyzer (NA1500, Fisons Instruments). Sequential extraction methods were applied to characterize solid P speciation (Ruttenberg 1992) and solid Fe speciation (Poulton and Canfield 2005). The steps involved in these methods are listed in Table 1. A distinction was made in sediment Fe(II), involving pyrite and carbonate-Fe, and sediment Fe(III), comprising Fe-oxides. The sum of the Fe(II) and Fe(III) fractions was defined as the root reactive iron (RR-Fe) fraction. Pyrite (FeS2) was determined separately using X-ray diffraction (Qmineral, Heverlee, Belgium). All geochemical analyses were carried out for 5 replicates per sediment type, except for the XRD analysis (1 replicate per sediment type).
Statistical analyses
To identify the main effects and interaction effects of the fixed factors sediment type, plant species and time of harvest on the response variables N, P, K, and Fe concentrations in roots and leaves, we used univariate, general linear models that included these main effects and the species x sediment interaction effect. More specifically, we analyzed how species differed in the uptake of N, P, K, and Fe in general, and how uptake was mediated by sediment type. Moreover, as the sediment types differed in Fe concentration, we examined whether plant growth decreased with increasing Fe concentration in the sediment. In principle, this analysis could be performed with a two-way ANOVA model design. We observed, however, that elemental concentrations in the plant gradually declined over the course of the experiment. To correct for this effect, we therefore developed a general linear model that also included time of harvest as a fixed factor. Moreover, the previously reported variation in the plant species tolerance to iron toxicity (Laan et al. 1991; Snowden and Wheeler 1995) motivated the inclusion of a species x sediment interaction effect in the models. As these fixed factors are orthogonal, effect sizes were determined using type III sum of squares. Before each model-run, the dependent variable – i.e. tissue concentrations of N, P, K, and Fe – was tested for homogeneity of variances with a Levene’s test (p > 0.05). When a dependent variable did not meet the assumption of homogeneity of variance, the log-transformed variable was used if this improved the homogeneity of variances as quantified by the Levene test statistic. We note, however, that a univariate general linear model is robust for departures from homogeneity of variance when the sample sizes are more or less equal (Box 1954). In contrast, alternative non-parametric procedures (such as the Kruskal-Wallis H test followed by a Dunn’s post hoc test) have very low power and associated high type II error rates, especially for our type of study design with relatively low replication within groups (e.g. Day and Quinn 1989). Therefore, when a dependent variable did not meet the assumption of homogeneity of variances after log-transformation, the output of the model could still be interpreted because the sample sizes between groups were equal in this experiment. Differences between groups were analyzed using least square difference tests (p < 0.05) following Webster (2007). The same post hoc test was used to assess geochemical differences between sediment types using the geochemical variables analysed on the field sediment samples. All statistical analyses were carried out in SPSS 22.0.0.1.
Results
Iron and nutrient concentrations in the sediment types
Table 2 lists the composition of macronutrients and iron present in the four sediment types used in this study. These sediments differed significantly in chemical composition (F(39, 12.6) = 62.05, p < .0005; Wilk”s Λ < .0005, partial η2 = 0.994). The southern, well consolidated, Zuiderzee Holocene-deposit of Markermeer material contained the highest concentration of RR-Fe and Fe-P and is hereafter referred to as MH, while the soft mud from the north of the lake contained the lowest concentration of RR-Fe and Fe-P and is hereafter referred to as ML. Soft mud from the south (on top of the Zuiderzee deposit) had RR-Fe and Fe-P concentrations in between MH and ML (hereafter referred to as MM)
The total phosphorus concentration ranged from 1191 mg kg−1 in MH to 361 mg kg−1 in ML. Iron-bound phosphorus was the dominant P fraction in MH and MM, whereas P in crystalline apatite was the dominant P fraction in ML. The amount of N present in the Markermeer sediments ranged from 2833 mg kg−1 in MM to 1611 mg kg−1 in ML. The nitrogen concentrations between MH and MM did not differ significantly, but both were higher than ML (p < 0.01). Potassium ranged from 4579 mg kg−1 in MH to 2619 mg kg−1 in ML, and all Markermeer sediments had significantly different K concentrations (p < 0.05).
Main effects on plant tissue composition
Table 3 shows how sediment type, plant species identity and time of harvest affected the average N, P, K, and Fe concentrations in leaves and roots. For the average N, P, K, and Fe concentrations in the leaves, no significant differences were observed between the Markermeer sediment types. Only the leaf P concentrations in MH and MM were significantly higher than in ML. For the roots, more significant differences were found for the average concentration of P, K and Fe, however, no differences were observed for root N. The root P concentration was significantly highest in MH, followed by MM and ML. The root K concentration was also highest in MH, but this was only significantly higher compared to MM. The highest root Fe concentration was found in ML.
Eupatorium cannabinum had lower N, P, and K concentrations in both the leaves and the roots, compared to R. maritimus and P. australis (Table 3). The latter two plants species were not significantly different, except for leaf N, where the average concentration in P. australis was significantly higher. Although nutrient concentrations did not show large differences, the Fe concentrations in both leaf and root showed clearly different patterns: The leaf Fe concentration was significantly highest in E. cannabinum, while the Fe concentration in roots was significantly highest in P. australis.
The leaf N, P, and K concentrations significantly decreased throughout the experiment: the highest concentrations were measured after 28 days and the lowest concentrations after 84 days (Table 3). Again, the Fe concentrations showed a contrasting pattern, both leaf and root Fe concentrations were more constant over time.
Root surface composition
Qualitative analysis of root surface composition showed that iron plaque was substantial on roots of P. australis (Fig. 2). Some iron plaque was visible on roots of R. maritimus, while none could be discerned on roots of E. cannabinum.
For P. australis, iron plaque was absent on roots grown in the control sediment and interestingly, the composition of the plaque differed among the Markermeer sediment types (Fig. 3). Potassium was more pronounced in iron plaque on roots of P. australis grown in ML as compared to the other two Markermeer sediments. Little co-precipitation of P occurred on roots of P. australis grown in MM, while a substantial amount was precipitated on roots grown in MH. In general, the higher the concentration of Fe(III) and Fe-P in the sediment, the more P co-precipitated with Fe on the roots.
Biomass production
Aboveground biomass production was significantly lower in all three Markermeer sediments as compared to the control sediment (Fig. 3a). Of the three Markermeer sediment types, aboveground biomass production of the iron-tolerant species R. maritimus and P. australis was highest in MM, followed by ML and MH. For the iron-intolerant species E. cannabinum this was the other way around, the highest biomass was obtained in ML, followed by MM and MH.
Root growth of E. cannabinum was reduced in all three Markermeer sediments compared to the control sediment, while for the other two plant species root growth was higher or similar to the control in MM and ML (Fig. 3b). For R. maritimus, belowground biomass production was highest in ML, whereas for P. australis root growth was highest in both MM and ML.
Species-specific effects of sediment type
Due to the presence of iron plaque on roots of P. australis (Fig. 2), it is of particular interest to examine how the nutrient balance of this plant species changed relative to R. maritimus and E. cannabinum from ML to MM to MH. These so-called sediment x species interaction effects are presented in Table 4, considering concentrations of N, P, K, and Fe as well as the N:P ratio in both leaves and roots.
Not many significant differences were found when looking at the root and leaf N and K concentrations (Table 4). However, the leaf P concentrations in R. maritimus and E. cannabinum were significantly lowest in ML and highest in MH, but did not differ significantly in P. australis. In contrast, all three plant species showed significantly lowest root P concentrations in ML and significantly highest root P concentrations in MH. As a result of these differences in leaf P and root P concentrations between the sediment types, significantly highest leaf N:P ratios for leaves and roots were found in ML. However, this trend was not significant for the leaf N:P ratio in P. australis, as it kept its leaf P concentration relatively stable. These results indicate that sediment type influences the nutrient balance in P. australis in a different way compared to R. maritimus and E. cannabinum due to the presence of iron plaque on roots.
No clear species differences were found in leaf or root Fe when growing on the different sediment types (Table 4).
N:P ratio in roots vs. leaves
Figure 4 shows scatter plots of N, P, and the N:P ratio in both roots and leaves at experimental time t = 28, 56, and 84 days. The sediment types were treated separately, whereas the plant species and time of harvest were grouped together. No clear relations could be discerned between the N:P ratio of roots and leaves, which is especially evident in MM and MH (Fig. 4a). The leaf N:P ratio shows that the majority of data points indicate N limitation, whereas some indicate N-P co-limitation or P limitation.
Scatter plots depicting the relation between N:P ratios in roots vs leaves (a), total content of N vs P in roots, leaves and flowers (b), N:P ratios in leaves vs concentration of N in leaves and flowers (c), N:P ratios in leaves vs P in leaves and flowers (d), N:P ratios in roots vs concentration of N in roots (e), N:P ratios in roots vs P in the roots (f). Sediment types include the southern Zuiderzee deposit (MH), the soft clay-rich layer on top of this deposit (MM), and the northern soft clay-rich layer (ML)
Figure 4b presents the total uptake of N and P and indicates that the relation between total N and P uptake did not differ at the three time-steps measured. This figure also shows that the total uptake of P was higher in MH than in ML, relative to the total uptake of N (and vice versa).
The N:P ratios of leaves relative to the concentrations of N in leaves and flowers did not show a trend for any of the three sediment types (Fig. 4c). The same was observed when comparing the N:P ratios of roots with the concentrations of N in the roots (Fig. 4e). As a consequence, when comparing the leaf and root N:P ratios with the P concentrations a negative trend was observed, with MH having relatively low N:P ratios with high concentrations of P and ML having relatively high N:P ratios with low concentrations of P (Fig. 4d,f).
Discussion
This experiment aimed to study the effects of iron toxicity in plants when grown in the iron-rich sediments of lake Markermeer. Therefore, we determined the presence and composition of iron plaque on the plant roots and measured the N, P, K, and Fe concentrations in both leaves and roots. The wetland species used in this study showed different responses to sediment type in terms of biomass production, iron plaque formation and N:P stoichiometry. In this section, we first discuss the presence and composition of iron plaque, after which we discuss the associated stoichiometric effects and its impacts on growth. Lastly, we briefly evaluate the implications for eco-engineering.
Presence and composition of iron plaque
Analysis of root surface composition showed that only P. australis produced substantial amounts of iron plaque on all three Markermeer sediments. This plant species is known for its high radial oxygen loss compared to other wetland species (Brix et al. 1996; Dickopp et al. 2011; Tercero et al. 2015) and, as a consequence, causes iron to be precipitated on plant roots (St-Cyr and Crowder 1989). Iron plaque damages the epidermis surface of roots (Jørgenson et al. 2013), which could explain the high Fe concentrations in the roots in our experiment (Table 3). Moreover, the Fe-P concentration of the sediment determined the composition of the iron plaque on the plant roots of this species. Phosphorus co-precipitated with Fe in high Fe-P sediment, while K in iron plaque was observed in low Fe-P sediment. The presence of K is explained by leakage of this element from the root during drying. This is explained by Snowden and Wheeler (1995), who only observed K leakage during drying when phosphorus did not co-precipitate with Fe.
No substantial amounts of iron plaque were formed on the roots of R. maritimus, which was unexpected because earlier studies showed that radial oxygen loss of R. maritimus was high (Laan et al. 1989, 1990). However, when the diffusion path length of the root increases, radial oxygen loss can decrease ten-fold (Laan et al. 1990), which might explain the lack of iron plaque on R. maritimus in this study. The Fe-intolerant E. cannabinum did not produce any iron plaque on its roots which is in concordance with the findings of Snowden and Wheeler (1995).
Stoichiometric effects
Iron plaque induces a form of indirect iron toxicity by acting as a barrier against iron, which in turn has adverse effects on the nutrient uptake and translocation mechanisms in the plant (Snowden and Wheeler 1995; Xu et al. 2009; Tripathi et al. 2014). The Fe-P concentration of the sediments used are positively correlated to P concentrations in the roots and ranged from 40 mg kg−1 in ML to 815 mg kg−1 in MH (Table 2). However, the P concentrations in the leaves of P. australis did not follow the P concentrations in the roots, probably because the iron plaque produced on the roots inhibited translocation of P to the shoot. As a result, root N:P ratios were lower in individuals grown on high Fe-P sediment, whereas leaf N:P ratios were not differing between individuals grown on high vs low Fe-P sediment. These results corroborate the findings of Snowden and Wheeler (1995) and Xu et al. (2009), who argued that plants that favor co-precipitation of P induce a higher uptake of P but severely reduce the translocation of P to the shoot.
Variation in leaf N concentrations was not large (interspecific and between-treatment average concentrations ranged between 1.59–3.39% dry wt), whereas leaf P concentrations varied more (0.11–0.55% dry wt). Nitrogen concentrations are well above the critical concentrations for N limitation (c. 1.4% cf. De Wit et al. 1963) and leaf P concentrations are also above that for P limitation (c. 0.07% cf. De Wit et al. 1963). N:P ratios of most of our leaf samples indicate slight to moderate N-limitation (< 14 cf. Olde Venterink 2011). Variation in N:P ratios in both leaves and roots was mainly caused by the variability in the concentration of P rather than N. This is because the homeostatic regulatory mechanisms of herbaceous plants result in relatively stable N concentrations (Güsewell 2004). In the current study, this was most evident in R. maritimus, an Fe-tolerant species that was, unlike the other two species, able to translocate more P to its leaves under Fe-P-rich conditions. For this species, iron plaque formed only in marginal amounts and did not reduce the translocation of P, which resulted in relatively low leaf N:P ratios ranging from 3.8 in MH to 12.4 in ML. These observations indicate that the sediment Fe-P fraction not only influences P concentrations in the roots, but also the P concentrations in the leaves. Moreover, no clear relationship could be discerned between the N:P ratio in leaves and the N:P ratio in roots for all three test species, especially at medium and high Fe-P rich conditions. These observations suggest that both inter- and intraspecific variation in iron plaque formation determines the uptake of P, but low plasticity (e.g. the ability of a species to increase or decrease the uptake according to its needs) in N uptake prevents Fe-tolerant species to profit from their more effective P acquisition as the intraspecific variation in N concentrations was low. Due to these stoichiometric effects, we argue that the leaf N:P ratios of plants grown in iron-rich sediments may not be a good proxy for discerning the type of nutrient limitation.
Impacts on growth
We found clear impacts of Fe-P sediments on biomass production for all three wetland species, which is partly related to iron plaque and its associated stoichiometric effects described in the previous sections. Compared with the control sediments, all species showed reduced aboveground growth, whereas R. maritimus and P. australis showed higher root production than the control in at least one sediment type (R. maritimus in ML and P. australis in ML and MM). From a nutrient perspective, it makes sense that dry matter allocation is favored to roots when grown in sediment containing relatively low amounts of nutrients. However, the reduced aboveground biomass production in the nutrient-rich MH suggests otherwise, as in high nutrient conditions dry matter allocation should be favored to the shoot (Ericsson 1995; Shipley and Meziane 2002). In the high Fe-P sediment all plant species showed strong growth reduction, aboveground as well as belowground compared with the control sediments. Since only P. australis produced iron plaque, we may assume that indirect iron toxicity as a consequence of iron plaque was not the cause of reduced growth. Moreover, R. maritimus – although Fe tolerant and able to transport more P to its leaves when grown in high Fe-P sediment – appears to be as strongly limited in growth in high Fe-P sediment as the other two species. These observations do not support the hypothesis that indirect Fe toxicity in which iron plaque formation disrupts a balanced N and P acquisition is the major problem for our test plants. Our results also raise the question as to whether N and/or P are primary limiting factors for plant growth in these sediment types at all. The significantly raised Fe concentrations compared with the control sediments in the leaves of our three test species do indicate that direct Fe toxicity following the uptake of Fe cannot be excluded as a mechanism potentially explaining inhibited aboveground growth. Application of dolomite to these sediments could mitigate Fe-toxicity as it decreases the exchangeable soil Fe2+ (Suriyagoda et al. 2016).
Implications for eco-engineering
Initial high belowground biomass production is especially important when building wetland ecosystems from soft mud, as roots have the ability to stabilize sediments and reduce sediment degradation. This is also true for aboveground biomass as leaves and stems dampen shear stress on the substrate by attenuating waves (Nepf 2012). Examples where plants are used successfully to stabilize sediment include tidal flats (Ganthy et al. 2011), riparian zones (Edmaier et al. 2011) and salt marsh creeks (Chen et al. 2012).
The results presented in this study suggest that of the iron-rich sediments collected in Markermeer, ML is the best building material for constructing approximately 10,000 ha of wetland. It is foreseen by the building consortium to use wetland plants as eco-engineers to strengthen the soft mud and to speed up sediment consolidation and soil formation. We showed that based on the three commonly occurring wetland species in The Netherlands used in this study, R. maritimus is the best choice on the short-term because it has the potential to strengthen and stabilize the sediment at a faster pace than P. australis while at the same time having a fairly high aboveground biomass production.
Given the results presented in this study, we argue that the Fe(III) and Fe-P fraction in sediments should be explicitly considered when studying plant performance and nutrient dynamics in pioneer ecosystems. Especially in eco-engineering projects where wetlands are to be constructed by using iron-rich sediments and where plants are used as ecological engineers important soil characteristics should be measured and plant response should be monitored prior to deciding which building material and plant species to use.
Conclusions
This study shows that the production of iron plaque on plant roots is highly plant-species specific. Only Phragmites australis produced significant amounts of iron plaque, its composition mainly depending on the Fe(III) and iron-bound phosphorus concentration of the sediment. Plaque formation caused stoichiometric imbalances in leaves, suggesting an inhibited translocation of P to the leaves. Since all our test species showed inhibited aboveground growth, irrespective of iron plaque formation, we conclude that indirect iron toxicity and a disrupted N:P stoichiometry as a consequence of iron plaque was unlikely to be the cause of reduced growth. Thus, direct Fe toxicity following the uptake of Fe cannot be excluded as a mechanism potentially explaining our results, but needs further examination in longer-term experiments. Our results stress the importance of considering the Fe(III) and Fe-P fraction in sediments, especially in eco-engineering projects where plants are used as ecological engineers for speeding up sediment consolidation and soil formation.
References
Ayeni O, Kambizi L, Laubscher C, Fatoki O, Olatunji O (2014) Risk assessment of wetland under aluminium and iron toxicities: a review. Aquat Ecosyst Health Manag 17:122–128
Blom CWPM (1999) Adaptations to flooding stress: from plant community to molecule. Plant Biol 1:261–273
Box GEP (1954) Some theorems on quadratic forms applied in the study of analysis of variance problems. Ann Stat 25:290–302
Brix H, Sorrell BK, Schierup HH (1996) Gas fluxes achieved by in situ convective flow in Phragmites Australis. Aquat Bot 54:151–163
Chen Y, Thompson CEL, Collins MB (2012) Saltmarsh creek bank stability: Biostabilisation and consolidation with depth. Cont Shelf Res 35:64–74
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
De Lucas Pardo MA, Bakker M, Van Kessel T, Cozzoli F, Winterwerp JC (2013) Erodibility of soft freshwater sediments in Markermeer: the role of bioturbation by meiobenthic fauna. Ocean Dyn 63:1137–1150
De Wit CT, Dijkshoorn W, Noggle JG (1963) Ionic balance and growth of plants. Verslagen van Landboukundige Onderzoeken 69.15. Pudoc, Wageningen
Dickopp J, Kazda M, Cízková H (2011) Differences in rhizome aeration of Phragmites Australis in a constructed wetland. Ecol Eng 37:1647–1653
Edmaier K, Burlando P, Perona P (2011) Mechanisms of vegetation uprooting by flow in alluvial non-cohesive sediment. Hydrol Earth Syst Sci 15:1615–1627
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annu Rev Environ Resour 30:75–115
Ericsson T (1995) Growth and shoot: root ratio of seedlings in relation to nutrient availability. Plant Soil 168:205–214
Ganthy F, Sottolichio A, Verney R (2011) The Stability of Vegetated Tidal Flats in a Coastal Lagoon Through Quasi In-Situ Measurements of Sediment Erodibility. Journal of Coastal Research, SI 64. Proceedings of the 11th International Coastal Symposium, pp 1500–1504
Geurts JJM, Sarneel JM, Willers BJC, Roelofs JGM, Verhoeven JTA, Lamers LPM (2009) Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: a mesocosm experiment. Environ Pollut 157:2072–2081
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Jørgenson KD, Lee PF, Kanavillil N (2013) Ecological relationships of wild rice, Zizania spp. 11. Electron microscopy study of iron plaques on the roots of northern wild rice (Zizania Palustris). Botany 91:189–201
Laan P, Smolders A, Blom CWPM, Armstrong W (1989) The relative roles of internal aeration, radial oxygen losses, iron exclusion and nutrient balances in flood-tolerance of Rumex species. Acta Botanica Neerlandica 38:131–145
Laan P, Tosserams M, Blom CWPM, Veen BW (1990) Internal oxygen transport in Rumex species and its significance for respiration under hypoxic conditions. Plant Soil 122:39–46
Laan P, Smolders A, Blom CWPM (1991) The relative importance of anaerobiosis and high iron levels in the flood tolerance of Rumex species. Plant Soil 136:153–161
Lamers LPM, van Diggelen JMH, den Op Camp HJM, Visser EJW, Lucassen ECHET, Vile MA, Jetten MSM, Smolders AJP, Roelofs JGM (2012) Microbial transformations of nitrogen, sulfur and iron dictate vegetation composition in wetlands: a review. Front Microbiol 3:1–12
LMRe (2014) Landelijk Meetnet Regenwater. Netherlands National Institute for Public Health and the Environment http://www.lml.rivm.nl/gevalideerd/. Accessed 17 Nov 2014
Mitsch WJ (1998) Ecological engineering – the 7-year itch. Ecol Eng 10:119–130
Nepf HM (2012) Flow and transport in regions with aquatic vegetation. Annu Rev Fluid Mech 44:123–142
Noordhuis R, Groot S, Dionisio Pires M, Maarse M (2014) Wetenschappelijk eindadvies ANT-IJsselmeergebied. Vijf jaar studie naar kansen voor het ecosysteem van het IJsselmeer, Markermeer en IJmeer met het oog op de Natura-2000 doelen. Open File Rep. 1207767–000, 98 pp
Odum HT, Odum B (2003) Concepts and methods of ecological engineering. Ecol Eng 20:339–361
Olde Venterink H (2011) Does phosphorus limitation promote species-rich plant communities? Plant Soil 345:1–9
Poulton SW, Canfield DE (2005) Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chem Geol 214:209–221
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37:1460–1482
Saaltink R, Dekker SC, Griffioen J, Wassen MJ (2016) Wetland eco-engineering: measuring and modeling feedbacks of oxidation processes between plants and clay-rich material. Biogeosciences 13:4945–4957
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16:26–331
Snowden RED, Wheeler BD (1993) Iron toxicity to fen plant species. J Ecol 81:35–46
Snowden RED, Wheeler BD (1995) Chemical changes in selected wetland plant species with increasing Fe supply, with specific reference to root precipitates and Fe tolerance. New Phytol 131:503–520
St-Cyr L, Crowder AA (1989) Factors affecting iron plaque on the roots of Phragmites australis (Cav.) Trin. Ex Steudel. Plant Soil 116:85–93
Suriyagoda LDB, Sirisena DN, Somaweera KATN, Dissanayake A, De Costa WAJM, Lambers H (2016) Incorporation of dolomite reduces iron toxicity, enhances growth and yield, and improves phosphorus and potassium nutrition in lowland rice (Oryza sativa L). Plant Soil. doi:10.1007/s11104-016-3012-0
Temmerman S, Meire P, Bouma TJ, Herman PMJ, Ysebaert T, de Vriend HJ (2013) Ecosystem-based coastal defence in the face of global change. Nature 504:79–83
Tercero MC, Álvarez-Rogel J, Conesa HM, Ferrer MA, Calderón AA, López-Orenes A, González-Alcaraz MN (2015) Response of biogeochemical processes of the water-soil-plant system to experimental flooding-drying conditions in a eutrophic wetland: the role of Phragmites australis. Plant Soil 396:109–125
Tripathi RD, Tripathi P, Dwivedi S, Kumar A, Mishra A, Chauhan PS, Norton GJ, Nautiyal CS (2014) Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics 6:1798–1800
Van der Welle MEW, Smolders AJP, Op den Camp HJP, Roelofs JGM, Lamers LPM (2007) Biogeochemical interactions between iron and sulphate in freshwater wetlands and their implications for interspecific competition between aquatic macrophytes. Freshw Biol 52:434–447
Van Kessel T, De Boer G, Boderie P (2008) Calibration suspended sediment model Markermeer. Open File Rep 4612:107
Vijverberg T, Winterwerp JC, Aarninkhof SGJ, Drost H (2011) Fine sediment dynamics in a shallow lake and implication for design of hydraulic works. Ocean Dyn 61:187–202
Webster R (2007) Analysis of variance, inference, multiple comparisons and sampling effects in soil research. Eur J Soil Sci 58:74–82
Wheeler BD, Al-Farraj MM, Cook RED (1985) Iron toxicity to plants in base-rich wetlands: comparative effects on the distribution and growth of Epilobium hirsutum and Juncus subnodulosus Schrank. New Phytol 100:653–669
Xu D, Xu J, He Y, Huang PM (2009) Effect of iron plaque formation on phosphorus accumulation and availability in the rhizosphere of wetland plants. Water Air Soil Pollut 200:79–87
Acknowledgements
This study was supported with funding from Netherlands Organization for Scientific Research (NWO), project no. 850.13.032 and the companies Boskalis and Van Oord. This manuscript was produced with unrestricted freedom to report all results. We want to thank Jerry van Dijk, the editor and two anonymous reviewers for helpful comments on the manuscript. We would also like to thank Utrecht Botanic Gardens for their help, support and advice during the greenhouse experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: N. Jim Barrow.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saaltink, R.M., Dekker, S.C., Eppinga, M.B. et al. Plant-specific effects of iron-toxicity in wetlands. Plant Soil 416, 83–96 (2017). https://doi.org/10.1007/s11104-017-3190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3190-4