Abstract
Background and aims
In many important viticultural areas of the Mediterranean basin, plants often face prolonged periods of scarce iron (Fe) availability in the soil. The objective of the present work was to perform a comparative analysis of physiological and biochemical responses of Vitis genotypes to severe Fe deficiency.
Methods
Three grapevine rootstocks differing in susceptibility to Fe chlorosis were grown with and without Fe in the nutrient solution.
Results
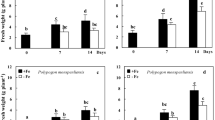
Rootstock 101-14, susceptible to Fe chlorosis, responded to severe Fe deficiency by reducing the root activity of phosphoenolpyruvate carboxylase (PEPC) and malate dehydrogenase (MDH), however, it accumulated high levels of citric acid. By contrast, rootstock 110 Richter, tolerant to Fe chlorosis, maintained an active metabolism of organic acids, but citric acid accumulation was lower than in 101-14. Similarly to 101-14, rootstock SO4 showed a strong decrease in PEPC and MDH activities. Nevertheless it maintained moderate citric acid levels in the roots, mimicking the response by 110 Richter.
Conclusions
Root PEPC and MDH activities can be used as tools for screening Fe chlorosis tolerance. Conversely, organic acids accumulation in roots may not be a reliable indicator of Fe chlorosis tolerance, particularly under conditions of severe Fe deficiency, because of their probable exudation by roots. Our results show that drawing sound conclusions from screening programs involving Fe deficiency tolerance requires short as well as long-term assessment of responses to Fe deprivation.
Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- CoA:
-
Coenzyme A
- CS:
-
Citrate synthase
- DW:
-
Dry weight
- EDTA:
-
Ethylenediaminetetraacetic acid
- FW:
-
Fresh weight
- MDH:
-
Malate dehydrogenase
- NADP+-IDH:
-
Isocitrate dehydrogenase
- PEPC:
-
Phosphoenolpyruvate carboxylase
- TCA:
-
Tricarboxylic acid
References
Bavaresco LE, Giachino E, Pezzutto S (2003) Grapevine rootstock effects on lime-induced chlorosis, nutrient uptake, and source-sink relationships. J Plant Nutr 26:1451–1465
Bavaresco L, van Zeller MI, Civardi S, Gatti M, Ferrari F (2010) Effects of traditional and new methods on overcoming lime-induced chlorosis of grapevine. Am J Enol Vitic 61:186–190
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brancadoro L, Rabotti G, Scienza A, Zocchi G (1995) Mechanisms of Fe-efficiency in roots of Vitis spp. in response to iron deficiency stress. Plant Soil 171:229–234
Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329:1–25
Chollet R, Vidal J, O’Leary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47:273–298
Covarrubias JI, Rombolà AD (2013) Physiological and biochemical responses of the iron chlorosis tolerant grapevine rootstock 140 Ruggeri to iron deficiency and bicarbonate. Plant Soil 370:305–315
Covarrubias JI, Pisi A, Rombolà AD (2014) Evaluation of sustainable management techniques for preventing iron chlorosis in the grapevine. Aust J Grape Wine Res 20:149–159
De Nisi P, Zocchi G (2000) Phosphoenolpyruvate carboxylase in cucumber (Cucumis sativus L.) roots under iron deficiency: activity and kinetic characterization. J Exp Bot 51(352):1903–1909
De Nisi P, Vigani G, Zocchi G (2010) Modulation of iron responsive gene expression and enzymatic activities in response to changes of the iron nutritional status in Cucumis sativus L. Available from Nature Precedings. doi:10.1038/npre.2010.4658.1
Donnini S, Castagna A, Ranieri A, Zocchi G (2009) Differential responses in pear and quince genotypes induced by Fe deficiency and bicarbonate. J Plant Physiol 166:1181–1193
Donnini S, Prinsi B, Negri AS, Vigani G, Espen L, Zocchi G (2010) Proteomic characterization of iron deficiency responses in Cucumis sativus L. roots. BMC Plant Biol 10:268
Foyer CH, Noctor G, Hodges M (2011) Respiration and nitrogen assimilation: targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J Exp Bot 62(4):1467–1482
Goldberg DM, Ellis G (1974) Isocitrate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. VerlagChemie/Academic Press, New York, pp 183–189
Jelali N, Wissal M, Dell’Orto M, Abdellya C, Gharsalli M, Zocchi G (2010) Changes of metabolic responses to direct and induced Fe deficiency of two Pisum sativum cultivars. Environ Exp Bot 68:238–246
Jimenez S, Gogorcena Y, Hévin C, Rombolà AD, Ollat N (2007) Nitrogen nutrition influences some biochemical responses to iron deficiency in tolerant and sensitive genotypes of Vitis. Plant Soil 290:343–355
Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS Lett 581:2273–2280
Lance C, Rustin P (1984) The central role of malate in plant metabolism. Physiol Veg 22(5):625–641
López-Millán AF, Morales F, Andaluz S, Gogorcena Y, Abadía A, De Las Rivas J, Abadía J (2000) Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiol 124:885–897
López-Millán AF, Morales F, Gogorcena Y, Abadía A, Abadía J (2009) Metabolic responses in iron deficient tomato plants. J Plant Physiol 166:375–384
López-Rayo S, Di Foggia M, Bombai G, Yunta F, Rodrigues-Moreira E, Filippini G, Pisi A, Rombolà AD (2014) Blood-derived compounds can efficiently prevent iron deficiency in grapevine. Aust J Grape Wine Res 21:135–142
Masclaux C, Valadier MH, Brugière N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211:510–518
Neumann G (2006) Root exudates and organic composition of plant roots. In: Luster J, Finlay R (eds) Handbook of methods used in rhizosphere research. Swiss Federal Research Institute WSL, Birmensdorf, 536 p
Nikolic M, Römheld V, Merkt N (2000) Effect of bicarbonate on uptake and translocation of 59Fe in two grapevine rootstocks differing in their resistance to Fe deficiency chlorosis. Vitis 39(4):145–149
Ollat N, Laborde B, Neveux M, Diakou-Verdin P, Renaud C, Moing A (2003) Organic acid metabolism in roots of various grapevine (Vitis) rootstocks submitted to iron deficiency and bicarbonate nutrition. J Plant Nutr 26(10&11):2165–2176
Rodríguez-Celma J, Lattanzio G, Grusak MA, Abadía A, Abadía J, López-Millán AF (2011) Root responses of Medicago truncatula plants grown in two different iron deficiency conditions: changes in root protein profile and riboflavin biosynthesis. J Proteome Res 10:2590–2601
Rombolà AD, Tagliavini M (2006) Iron nutrition of fruit tree crops. In: Abadía J, Barton L (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer, Berlin, pp 61–83
Rombolà AD, Brüggemann W, López-Millán AF, Tagliavini M, Abadía J, Marangoni B, Moog PR (2002) Biochemical responses to iron deficiency in kiwifruit (Actinidia deliciosa). Tree Physiol 22:869–875
Römheld V, Marschner H (1986) Mobilitation of iron in the rhizosphere of different plant species. Adv Plant Nutr 2:123–218
Smith F (1974) Malate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. VerlagChemie/Academic Press, New York, pp 163–175
Srere PA (1967) Citrate synthase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic, New York, pp 3–11
Tagliavini M, Rombolà AD (2001) Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur J Agron 15:71–92
Tagliavini M, Rombolà AD, Marangoni B (1995) Response to Fe-deficiency stress of pear and quince genotypes. J Plant Nutr 18(11):2465–2482
Vance CP, Stade S, Maxwell CA (1983) Alfalfa root nodule carbon dioxide fixation. I: association with nitrogen fixation and incorporation into amino acids. Plant Physiol 72:469–473
Wong KF, Davies DD (1973) Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J 131:451–458
Yunta F, Di Foggia M, Bellido-Díaz V, Morales-Calderón M, Tessarin P, López-Rayo S, Tinti A, Kovács K, Klencsár Z, Fodor F, Rombolà AD (2013) Blood meal-based compound. Good choice as iron fertilizer for organic farming. J Agric Food Chem 61:3995–4003
Zocchi G (2006) Metabolic changes in iron-stressed dicotyledonous plants. In: Abadía J, Barton L (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer, Berlin, pp 359–370
Acknowledgments
The authors gratefully acknowledge the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) of Chile and the Erasmus Mundus External Cooperation Window for Chile (Lot 17)-European Union Community for Doctoral Scholarships to José Ignacio Covarrubias.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yong Chao Liang.
Rights and permissions
About this article
Cite this article
Covarrubias, J.I., Rombolà, A.D. Organic acids metabolism in roots of grapevine rootstocks under severe iron deficiency. Plant Soil 394, 165–175 (2015). https://doi.org/10.1007/s11104-015-2530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2530-5